I Hirano et al. American Journal of Gastroenterology 2025; 120(7):p 1502-1510. Open Access! Effect of Esophageal Dilation History on Efficacy Outcomes in Patients With Eosinophilic Esophagitis Receiving Budesonide Oral Suspension
Methods: This post hoc analysis assessed data from a 12-week, randomized, double-blind, placebo-controlled phase 3 study (NCT02605837) of budesonide oral suspension (BOS) 2.0 mg twice daily in patients (n=318) aged 11–55 years with EoE and dysphagia. Coprimary efficacy outcomes were histologic (≤ 6 eosinophils per high-power field [eos/hpf]) and dysphagia symptom (≥ 30% reduction in Dysphagia Symptom Questionnaire scores from baseline) responses at week 12.
Key findings:
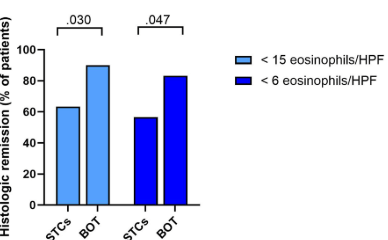
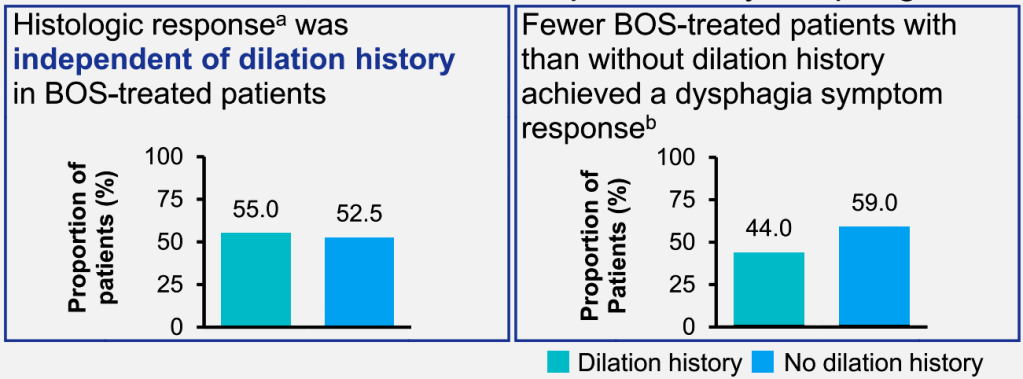
- Histologic responses (≤ 6 and < 15 eos/hpf) were similar regardless of dilation history
- Fewer BOS-treated patients with dilation history than no dilation history achieved a dysphagia symptom response (44.0% vs 59.0%)
Discussion Points:
- “Esophageal dilation may provide immediate relief from dysphagia (15); symptom improvement has been observed in 95% of dilated patients with EoE (29)…[however] dilation does not affect the underlying inflammation (18).”
- “A histologic response (<15 eos/hpf) to swallowed corticosteroids has also been associated with a reduced number of repeat esophageal dilations required to maintain a similar esophageal caliber compared with nonresponse (≥15 eos/hpf)…this supports swallowed corticosteroid use in patients who have undergone esophageal dilation, even in the absence of acute symptom improvement.”
- “Study limitations include potential enrollment of patients with severe disease due to stringent inclusion criteria.”
My take: While dilatation alone often improves symptoms, treatment with budesonide may help reduce need for repeat dilatations.
Related blog posts:
- Mechanical Dilation vs. Medical Therapy for Pediatric Eosinophilic Esophagitis
- Frequency of Strictures in Pediatric Eosinophilic Esophagitis
- Long-Term Treatment of Eosinophilic Esophagitis with Budesonide
- Eosinophilic Esophagitis: Once vs Twice Daily Steroid Treatment
- Impact of Disease Severity on Eosinophilic Esophagitis Treatment Responses
- ACG 2025 Guidelines for Eosinophilic Esophagitis
- “Tug” Sign For Eosinophilic Esophagitis and EoE Bowel Sounds Tips
- Budesonide for Maintaining EoE Remission
- Eosinophilic Esophagitis: Prevalence and Costs in the U.S.