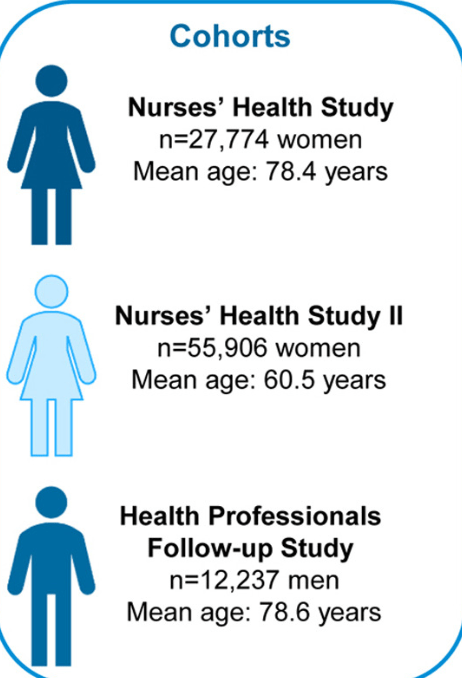
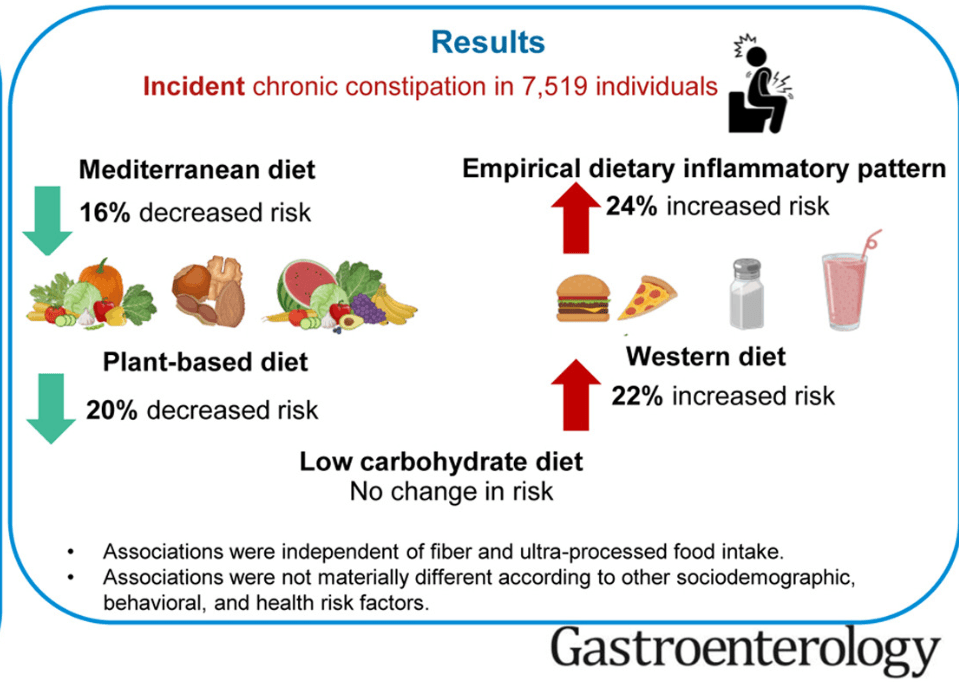
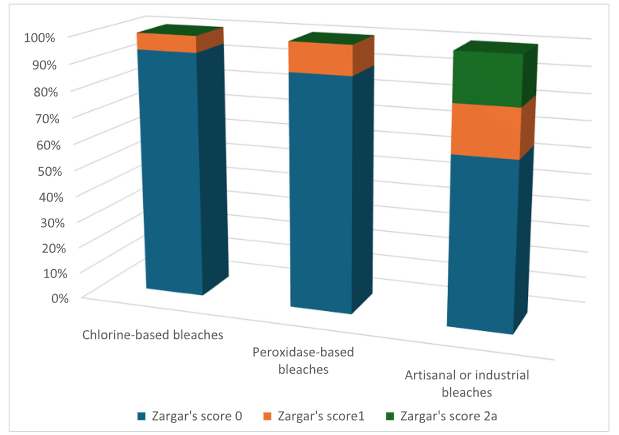
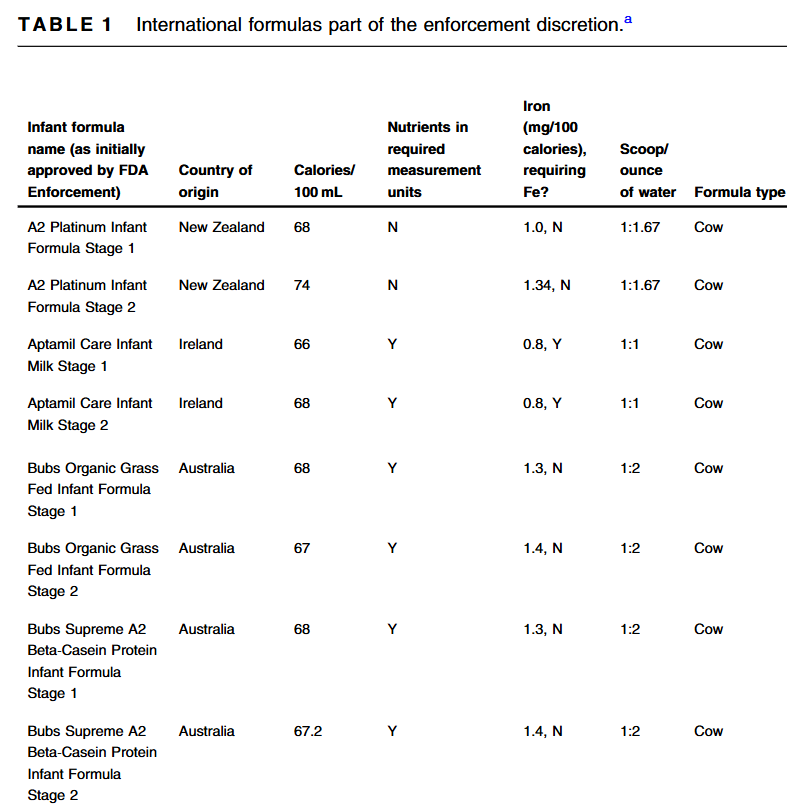
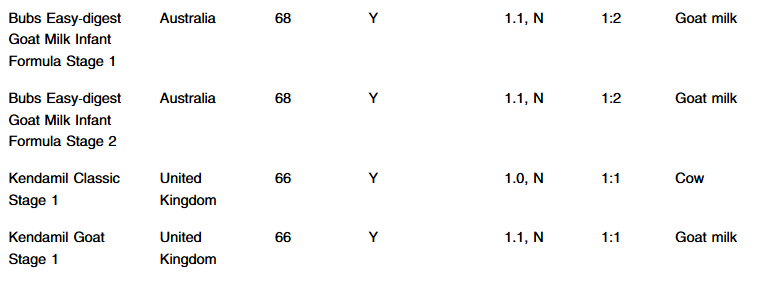
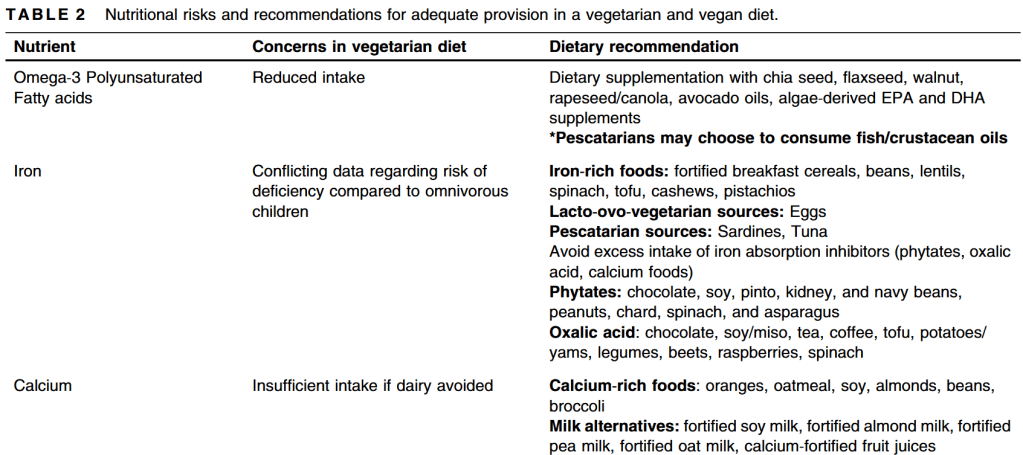
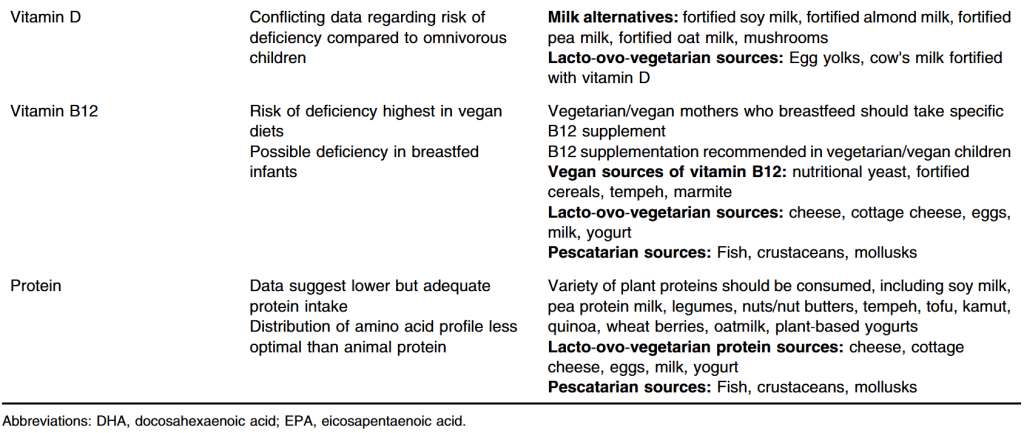
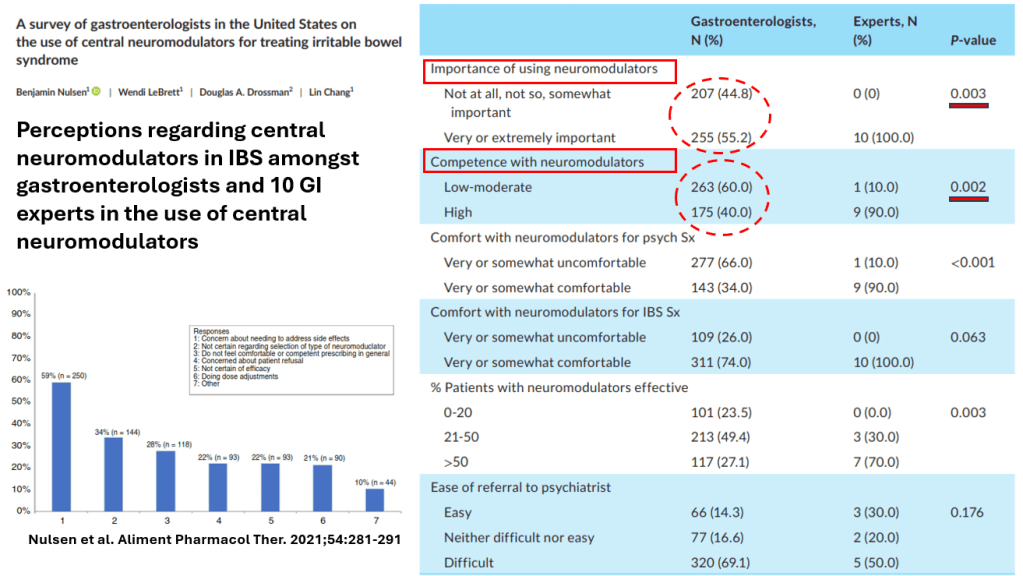
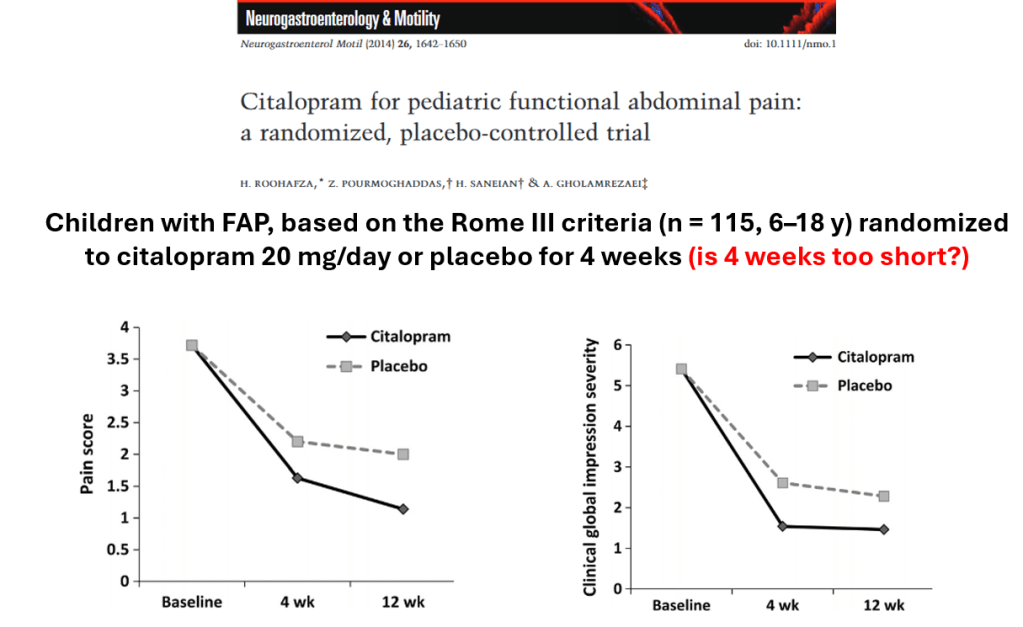
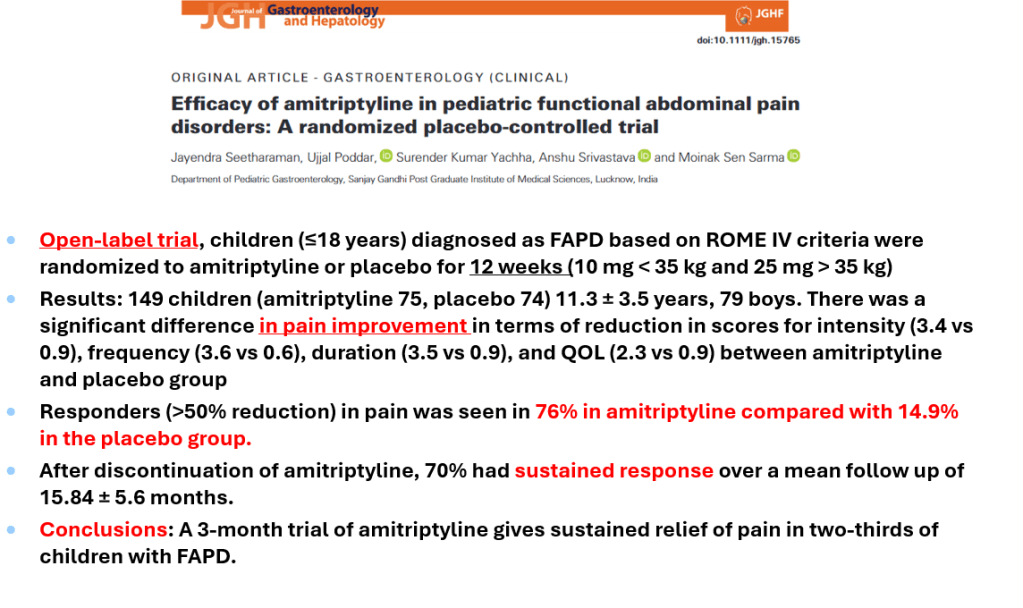
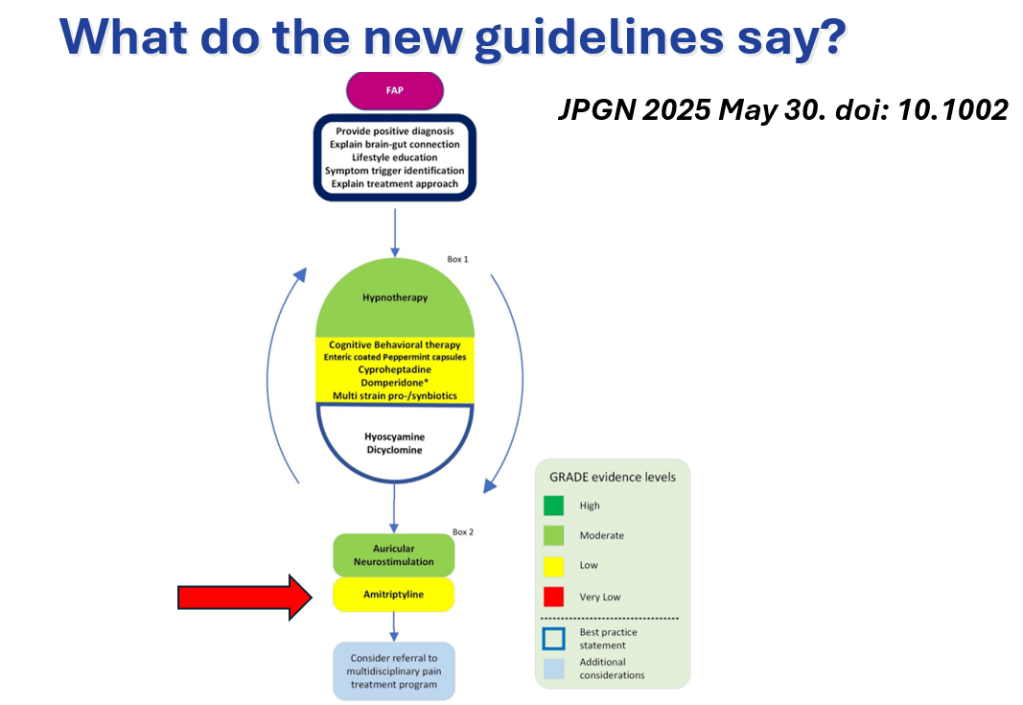
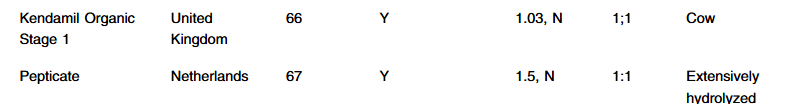S Hirsch et al. J Pediatr 2025;283:114628. Ten-Year Trends in Pharmacologic Management of Gastroesophageal Reflux Disease and Pediatric Feeding Disorders in Young Children
Methods: Single-center, retrospective cohort study of children less than 2 years (49,483) diagnosed with GERD or PFD (pediatric feeding disorder) between January 2014 and December 2023. Prescriptions were searched for proton pump inhibitors (PPI), H2-receptor antagonists (H2RA), cyproheptadine, erythromycin, metoclopramide, or prucalopride, and procedures were searched for intrapyloric botulinum injections.
Key findings:
- There was an increasing number of patients seen annually (6516 in 2014 vs 9109 in 2023)
- The percent of patients receiving any prescription for GERD or PFD declined by almost 50%, from 36.5% in 2014 to 18.7% in 2023 (P < .001)
- There was a particular decline in PPI prescriptions, with 25.3% of patients receiving PPI in 2014 and 7.1% receiving PPI in 2023 (P < .001)
- There was also a decline in H2RA prescriptions, with 17.0% of patients receiving H2RA in 2014 and 11.1% receiving H2RA in 2023 (P < .0001).
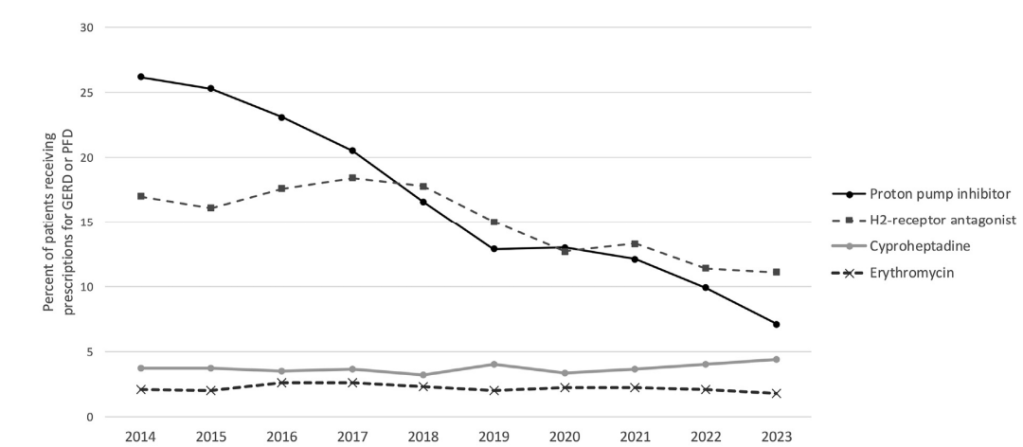
- In their discussion, the authors note that: “in contrast to the current findings, prior studies typically have shown increasing PPI prescriptions, with some of these studies demonstrating declining H2RA prescriptions (9-17)…. However, it is notable that 3 more recent international studies did demonstrate declining PPI prescriptions specifically in the final years of the study (18-20).”
- “Multiple studies have failed to demonstrate efficacy of acid suppression in infants with nonspecific gastroesophageal reflux symptoms, and there is no evidence that acid suppression affects feeding behaviors.(21-23)”
- “In addition, there has been growing concern about PPI side effects, which include increased infections, decreased bone density, and increased allergy development
including eosinophilic esophagitis, with numerous recent studies on these risks.(24-26)”
My take: I’ve been a big fan of the aerodigestive research from the pediatric GI group in Boston. This is another useful study showing less use of acid suppression, especially PPIs in young children and infants. This likely indicates better alignment of clinical practice with consensus recommendations that advise against acid suppression as first-line management in this population.
Related blog posts:
- Understanding Reflux/Airway Disease and Potential Role of Airway Impedance
- Arching in Infants Not Due to Reflux
- When Is It OK To Ignore Laryngeal Penetration?
- Incredible Review of GERD, BRUE, Aspiration, and Gastroparesis
- Good Episode of Bowel Sounds on Reflux
- Acid Suppression for Laryngomalacia -Handed This Article to My ENT Colleagues
- BRUEs in Boston –Two Punch Study
- Something Useful for Apparent Life-Threatening Events (ALTEs)
- Blaming Reflux for BRUEs -Not Changing Despite Guideline Recommendations
- Do Acid Blockers Given to Infants Increase the Risk of Allergic Disease?
- 2018 Pediatric Gastroesophageal Reflux Clinical Practice Guidelines
- Clinical evaluation not sensitive for aspiration
- How Many Kids with Reflux have Reflux?
- What I Don’t Like About a “Multidisciplinary Approach” for Infants with GERD-Like Symptoms
- Better to do a coin toss than an ENT exam to determine reflux
- Hard Data on Blenderized Diets
- Treating reflux does not help asthma | gutsandgrowth
- How Likely is Reflux in Infants with “Reflux-like … – gutsandgrowth
- Which kids who aspirate need a gastrostomy tube?