P Feuerstadt et al. AJG 2025; 120: 2468-2470. Treatment of Clostridioides difficile: The Times They Are Changing
This article summarizes the recent changes in the treatment options for Clostridioides difficile (C diff).
Key points:
- Fidaxomicin targets C diff with limited collateral microbiome disruption. This leads to significantly lower recurrence rates compared to vancomycin. Thus, it is preferred 1st line therapy for initial and recurrent C diff. In “the coming years, fidaxomicin is expected to come off patents” which will improve access and affordability.
- Bexlotoxumab which lowered recurrence rate is no longer being produced
- FMT via Openbiome is no longer available. In those in which FMT was used, options include the following:
- live-jslm (REBYOTA), a broad consortium enema-based formulation
- live-brpk (VOWST), a narrow consortium of Firmicutes in an encapsulated form. This treatment in adults: four capsules daily for three days
- Both treatments are not recommended for patients who are severely immunocompromised. In these patients, prolonged vancomycin course with taper or using every other day therapy with fidaxomicin for days 7-25 could be considered
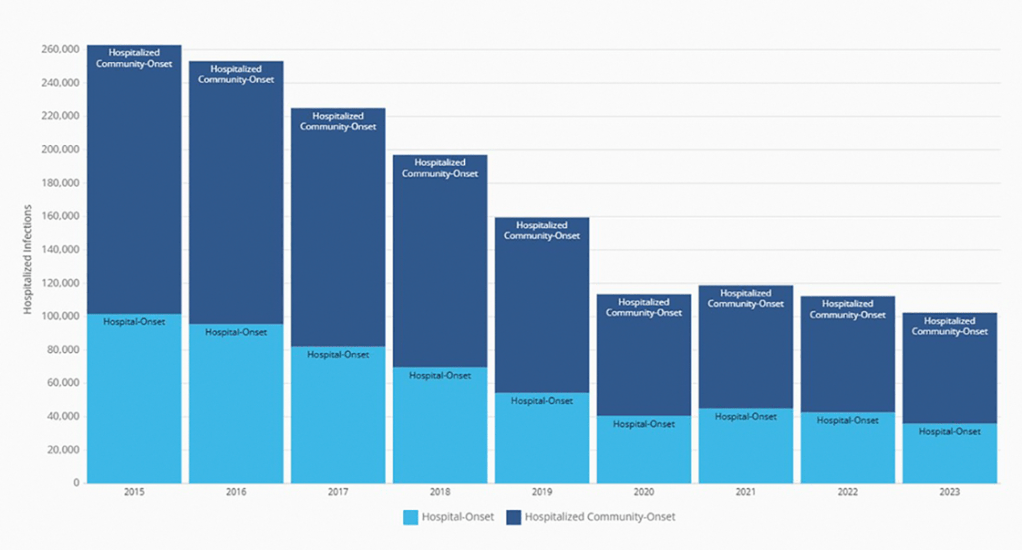
My take: I have been seeing less C diff cases recently. This may be due to better antibiotic stewardship, changes in C diff strains, or improved testing approaches.. My observation is supported by recent reports:
AY Guh et al. Infect Dis Clin North Am. 2025. 39:567-580. Changes in the Epidemiology of Clostridioides difficile Infection
Related blog posts:
- Fidaxomicin Treatment of Clostridioides difficile in Children and Adolescents
- C difficile three-fer: Overdiagnosis with Multiplex Testing, Fidaxomicin Pediatric Approval, & Changing Incidence (2020)
- Fidaxomicin Effective in Open-Label Pediatric Study (2014)
- “Diagnostic Stewardship” –Reducing Unnecessary Clostridioides difficile Treatment by Changing Testing Approach (2024)
- OpenBiome Suspending FMT Shipments (2024)
- ACG Clostridium Difficile Guidelines Plus One (2021)
- 4 Points for C diff in Inflammatory Bowel Disease
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.


