J Adler et al. Am J Gastroenterol 2025; 120: 1076-1086. HLA DQA1*05 and Risk of Antitumor Necrosis Factor Treatment Failure and Anti-Drug Antibody Development in Children’s with Crohn’s Disease.
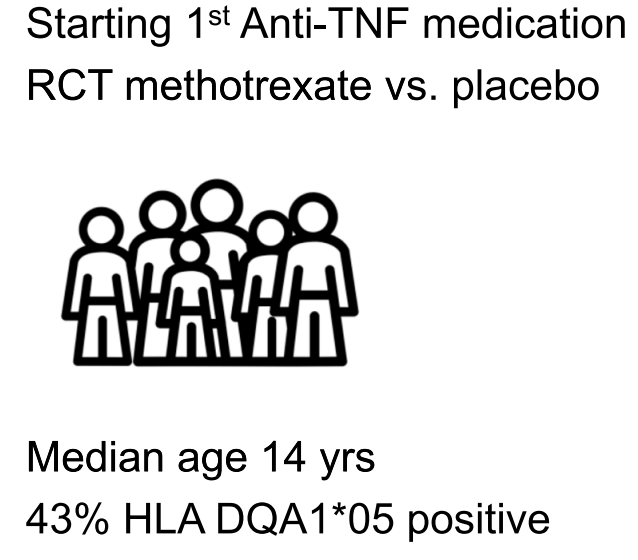
This was a prospective, double-blind, placebo-controlled trial with 204 patients examining the clinical outcomes of anti-TNF with or without methotrexate (COMBINE).
Key findings:
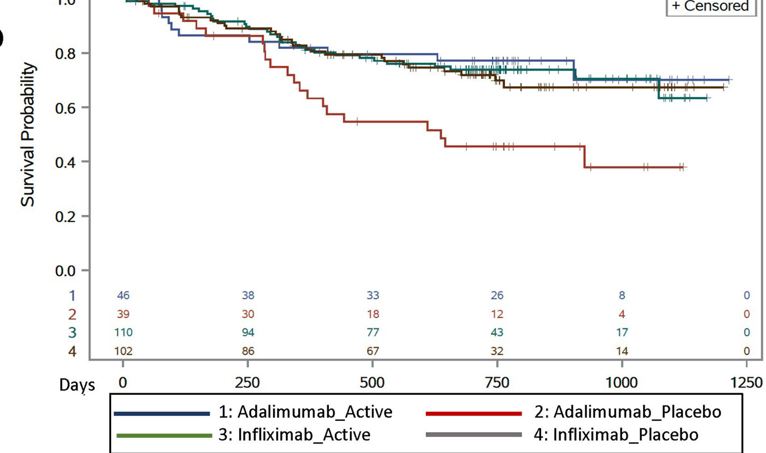
- Treatment failure in HLA DQA1*O5: A trend toward increased treatment failure among HLA DQA1*05-positive participants was not statistically-significant (hazard ratio 1.58; P = 0.08).
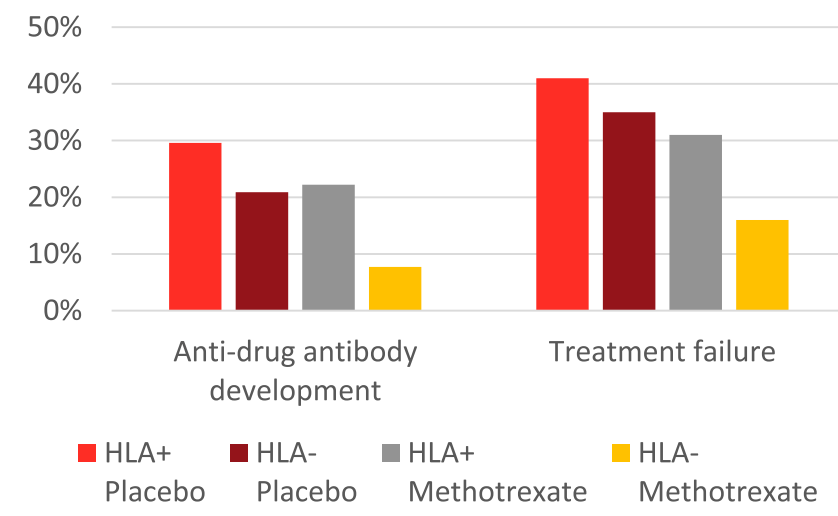
- HLA DQA1*05 and Treatment Failure Rate: During the followup period, HLA DQA1*05-positive patients had a 35% failure rate compared to 26% for those who were HLA DQA1*05-negative (P=0.098). The overall failure rate was 30%
- Methotrexate Combined with HLA DQA1*05 Effect: Patients who were HLA DQA1*05 negative and assigned to methotrexate experienced less treatment failures than HLA DQA1*05-positive patients on placebo (hazard ratio 0.31, 95% CI 0.13-0.70; P = 0.005).
- Anti-TNF Medication Comparison: Treatment failure was similar between infliximab and adalimumab, 29% and 33% respectively
- Antidrug antibodies (ADA): A trend toward increased ADA development among HLA DQA1*05-positive participants was not significant (odds ratio 1.96, P = 0.09). The addition of methotrexate to the treatment regimen mitigated the risk of treatment failure among individuals positive for HLA DQA1*05 and reduced the odds of developing ADA by 90%.
- Rate of ADA: “After further stratification, HLA DQA1*05-negative participants assigned to methotrexate were less likely to develop ADA relative to HLA DQA1*05-positive patients on placebo (odds ratio 0.12; P = 0.008).”

Discussion Points:
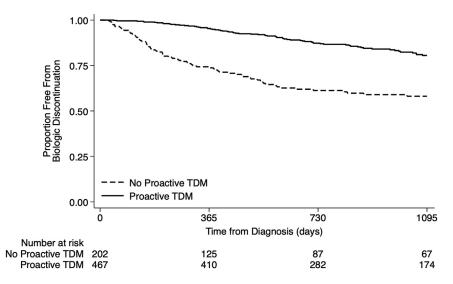
- “A retrospective by Fuentes-Valenzuela et al …found that if patients underwent proactive TDM, there was no increase in the rate of treatment discontinuation among those who were HLA DQ-A105 positive compared with HLA DQ-A105 negative”
- “The totality of evidence suggests that HLA DQ-A1*05 seems to confer a risk of both immunogenicity and treatment failure, particularly among infliximab-treated patients. Furthermore, this risk may be mitigated by the use of proactive TDM and/or concomitant immunomodulators.”
My take (borrowed in part from authors): “40% of patients were HLA DQ-A1*05 positive, which was associated with a trend toward increased risk of both treatment failure and ADA. These risks were mitigated, but not eliminated, by adding oral methotrexate.” The use of combination therapy (methotrexate with anti-TNF) was associated with the lowest failure rates.
Also, unrelated article: DA Carlson et al. Gastroenterology 2025; 168: 1114-1127. A Standardized Approach to Performing and Interpreting Functional Lumen Imaging Probe Panometry for Esophageal Motility Disorders: The Dallas Consensus. Congratulations to Dr. Garza from our group who was one of the authors
Related blog posts:
- Genetic Test to Help Determine Need for Combination Therapy with Anti-TNF
- COMBO-IBD Study -Combination Immunomodulator Use and Thresholds
- Why Do Children Taking Adalimumab Benefit from Methotrexate Dual Therapy?
- Combination Therapy Associated with Treatment Persistence
- Combination Therapy Study Points to Central Role of Adequate Drug Levels
- Can Therapeutic Drug Monitoring with Monotherapy Achieve Similar Results as Combination Therapy for IBD?
- Don’t be Fooled About Withdrawing Immunomodulator Cotherapy -Look Past the Headline
- Methotrexate Dosing in Dual Therapy