JA Murray, JA Syage et al.Gastroenterol 2022; 163: 1510-1521. Open access! Latiglutenase Protects the Mucosa and Attenuates Symptom Severity in Patients With Celiac Disease Exposed to a Gluten Challenge
Background: Latiglutenase (IMGX003) is an investigational dual-enzyme drug candidate that acts to degrade gluten in vivo when consumed with a meal. The authors note that “despite strict adherence to a GFD, about half of CD patients show evidence of persistent small intestinal mucosal injury (Marsh grades II–III);’ thus, there is a need to improve treatment with other measures in addition to diet.
Methods: 43 patients (IMGX003, n = 21; placebo, n = 22) completed this double blind and placebo controlled study which assessed the efficacy and safety of a 1200-mg dose of IMGX003 in patients with celiac disease (CD) exposed to 2 g of gluten per day for 6 weeks study
Key findings:
- In IMGX003-treated patients, there was less damage to mucosa. The mean change in the ratio of villus height to crypt depth (primary endpoint) for IMGX003 vs placebo was –0.04 vs –0.35 (P = .057). The mean change in the density of intraepithelial lymphocytes (secondary endpoint) for IMGX003 vs placebo was 9.8 vs 24.8 cells/mm epithelium (P = .018).
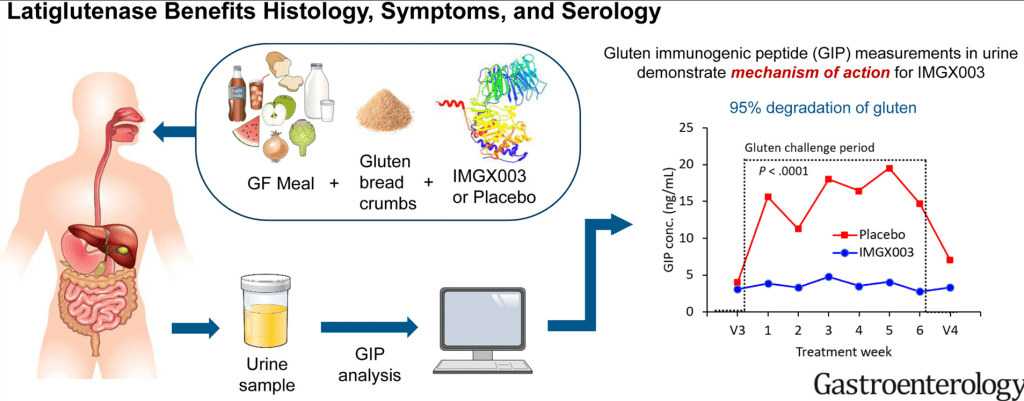
- Measurements of gluten-immunogenic peptides (GIP) in urine indicated 95% gluten degradation in the stomach by latiglutenase.
The 2 g dose per meal of gluten allowed used in the study, “would likely substantially exceed that accidently occurring while on a GFD, 4 supporting such an approach for management for gluten-triggered symptoms in treated patients.”
Graphical abstract:
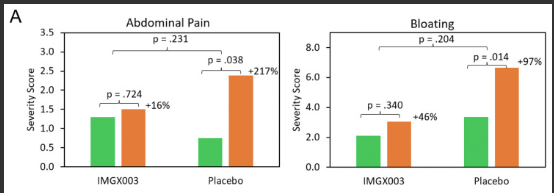
In both the placebo and IMGX003 groups, there was an increases in symptoms, but this was blunted in the treated group–Figure 2:
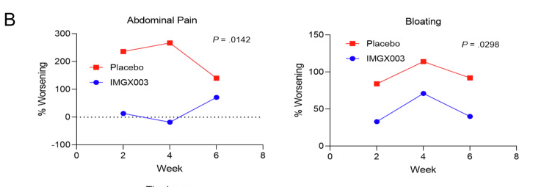
My take: This study shows the potential for latiglutenase to act as a ‘safety net’ to protect from CD from accidental gluten exposure. The findings reinforce the idea that this agent is not likely to be effective in the absence of gluten restriction. As an aside, I would be interested in finding out whether patients with presumed non-celiac gluten sensitivity would improve on this therapy.
Related blog posts:

