FH Mourad et al. Inflammatory Bowel Diseases, Volume 30, Issue 3, March 2024, Pages 459–469. Open Access! Are the New Biologics Effective in the Management of Postoperative Crohn’s Disease?
In this a systematic review, the authors identified 32 relevant studies. The literature review revealed some encouraging, although conflicting, results on the role of the newer biologic agents, ustekinumab and vedolizumab, in the prevention and treatment of postoperative Crohn’s disease. My take: More high-quality studies are needed to determine the effectiveness of these newer biologics at preventing recurrence disease activity after resection.
This is their recommended management algorithm:
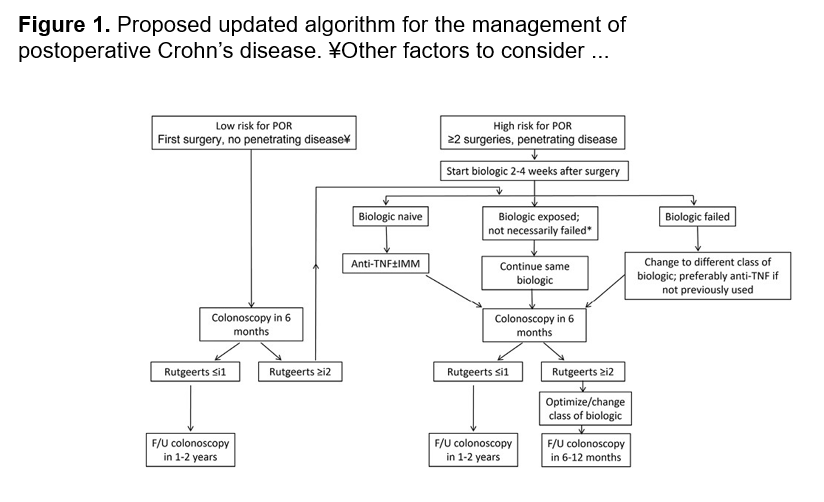
age of patient at diagnosis, and length of stricture.
—————————————
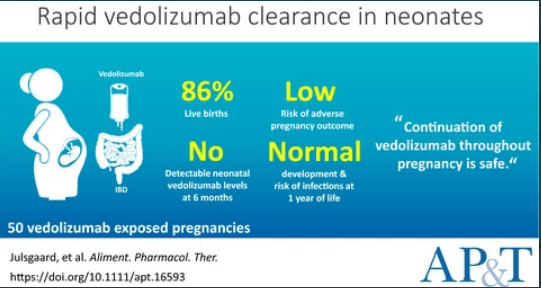
JH Gorodensky et al. Inflammatory Bowel Diseases, Volume 30, Issue 3, March 2024, Pages 496–498. Serious Infections in Offspring Exposed in Utero to Vedolizumab
Background/Methods: “Offspring exposed to vedolizumab in utero are born with lower blood drug (relative to maternal drug levels) compared with offspring exposed to TNFi or ustekinumab.4,5 In addition, offspring exposed to vedolizumab are known to clear the drug quickly after birth.6” In this IBM MarketScan database cohort of 8507 offspring to women with IBD, 43 offspring were exposed to vedolizumab. Key findings:
Key finding: The cumulative incidence of serious infection at 1 year was 2.3% in the vedolizumab group. This was lower than in those who had no drug exposure (3.0%) and similar to the rate in the TNFi (2.9%), traditional immunosuppressants (2.5%), and groups. Discussion: “This aligns with data from the PIANO study,5 a French retrospective cohort study,8 and the pan-European CONCEIVE study9“
My take: This article title is textbook clickbait. A more accurate title would be “Lack of Serious Infections in Offspring Exposed in Utero to Vedolizumab”
——————————————
J Lee et al. Inflammatory Bowel Diseases, Volume 30, Issue 3, March 2024, Pages 499–500. Intralesional Injections of a TNF-α Inhibitor to Treat Orofacial Granulomatosis
This case report demonstrated successful injection of orofacial granulomatosis in a 24 yo with moderate-to-severe CD of the small and large intestines who presented with facial edema, painful lip sores with crusting, and tongue fissures. After numerous other failed treatments (topical steroids, adalimumab, hydroxychloroquine, prednisone, and methotrexate), the patient who had a mild treatment response to certolizumab had three injections split between thigh and lower lip (intralesional). After improvement, the patient continued to maintain response with injections into the thigh.
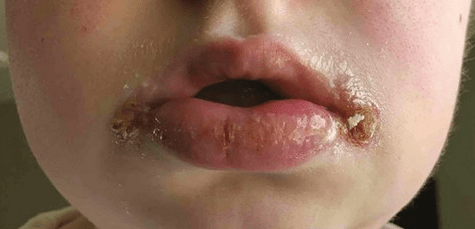
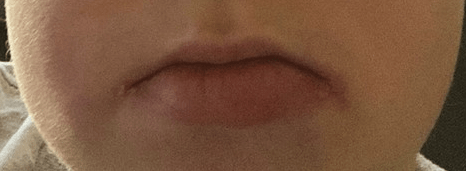
My take: This is an interesting case report; however, this is not an established therapy for orofacial granulomatosis.