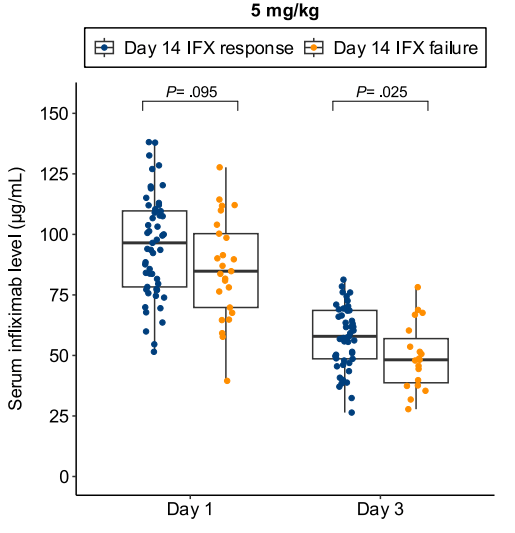
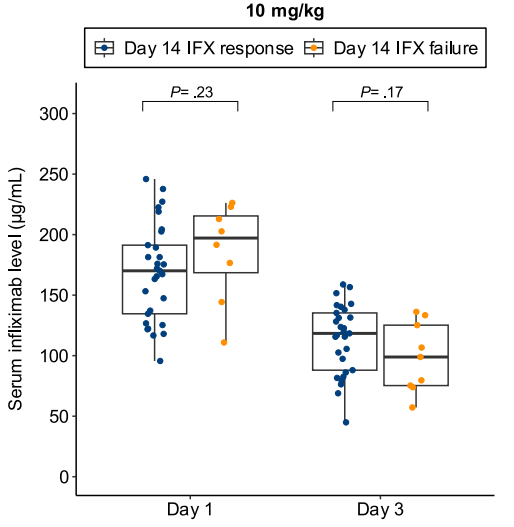
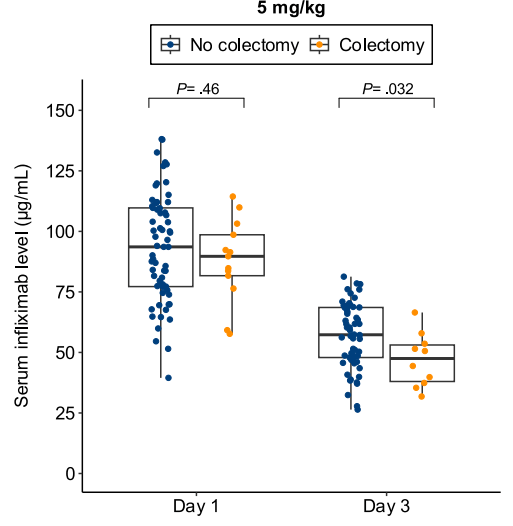
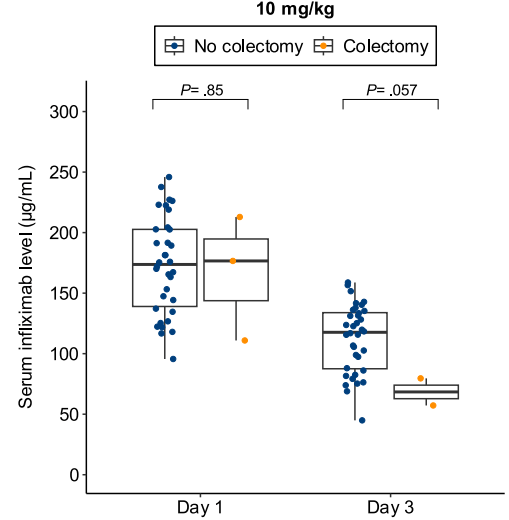
B Kang et al. Clinical Gastroenterology and Hepatology; 2026: 24: 201 – 209. Proactive Drug Monitoring Versus Clinically Based Dosing for Endoscopic Healing in Pediatric Crohn’s Disease Receiving Infliximab
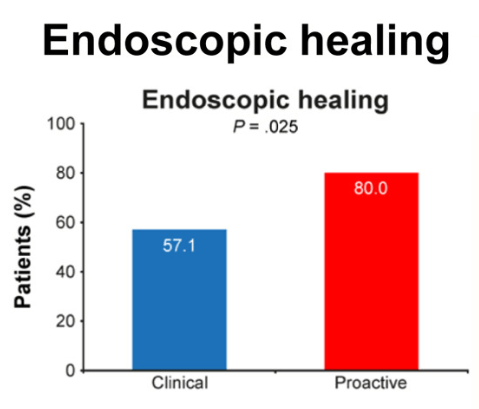
Methods: This was a non-blinded, randomized controlled trial of 112 biologic-naïve children with CD who had responded to IFX induction treatment at 4 centers in South Korea between July 2017 and November 2020. Patients were randomly assigned to receive dosing based on proactive TDM (proactive arm) or clinically based dosing (clinical arm). The primary endpoint was endoscopic healing (EH) at week 54.
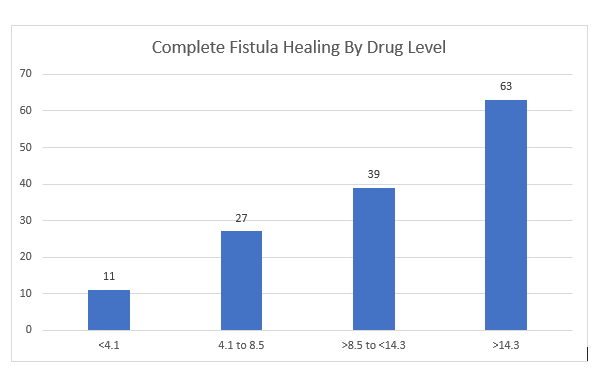
During the maintenance phase, patients received IFX 5 mg/kg every 8 weeks. In the proactive arm, treatment was intensified (shortening interval by 2- to 4-weeks) if trough level was less than 6 mcg/mL.
Key findings:
Discussion Points:
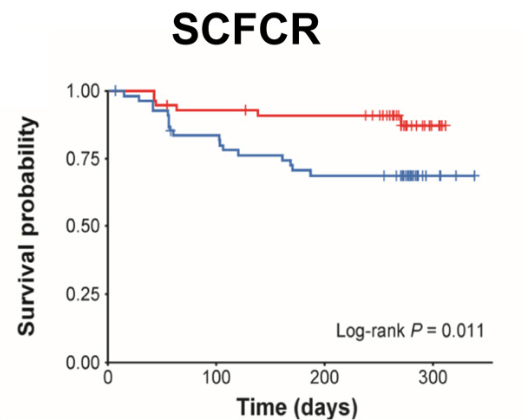
- “Our findings provide evidence that the proactive strategy resulted in increased EH rates and had a positive impact on SCFCR, biochemical remission, and FC, which serve as surrogate markers of EH.”
- “The PAILOT trial, the only prospective study on proactive TDM in pediatric patients with CD, demonstrated that proactive TDM with adalimumab resulted in higher SCFCR rates than reactive TDM (82% vs 46%; P < .001), consistent with our findings.8“
- “In our study, IMM [immunomodulator] modulation was performed in conjunction with proactive TDM, which may explain why no difference was observed in ADA development (proactive arm, 31.4% vs clinical arm, 28.6%. Proactive TDM has been confirmed to reduce the development of ADAs, and the concept of “optimized monotherapy” based on the view that proactive TDM effectively guides IMM withdrawal in combination therapy has been well-described.30 …in our institution, IMMs are discontinued as soon as possible after 1 year of combination therapy if adequate TDM is maintained.31“
My take: This study shows that proactive TDM is superior to clinical-based dosing. The findings may have been less pronounced if higher baseline doses of IFX were used. It is well-recognized that “standard” IFX (5 mg/kg/dose every 8 weeks) is usually insufficient in pediatric patients.
Related blog posts:
- Proactive Therapeutic Drug Monitoring and Better Outcomes in Pediatric Crohn’s
- NASPGHAN Pediatric Position Paper for Therapeutic Drug Monitoring Disease (2024)
- Why Pediatric Patients Need Higher Dosing of Infliximab
- Another Study Justifying Higher Infliximab Dosing in Pediatrics
- Proactive Therapeutic Drug Monitoring in Pediatric Crohn’s disease -Better Outcomes (2020)
- Here’s The Proof That Proactive Drug Monitoring Improves Outcomes in Children With Crohn’s Disease (2019). [This blog post summarizes reference #23 in the current study]
- Can Therapeutic Drug Monitoring with Monotherapy Achieve Similar Results as Combination Therapy for IBD? (2019)