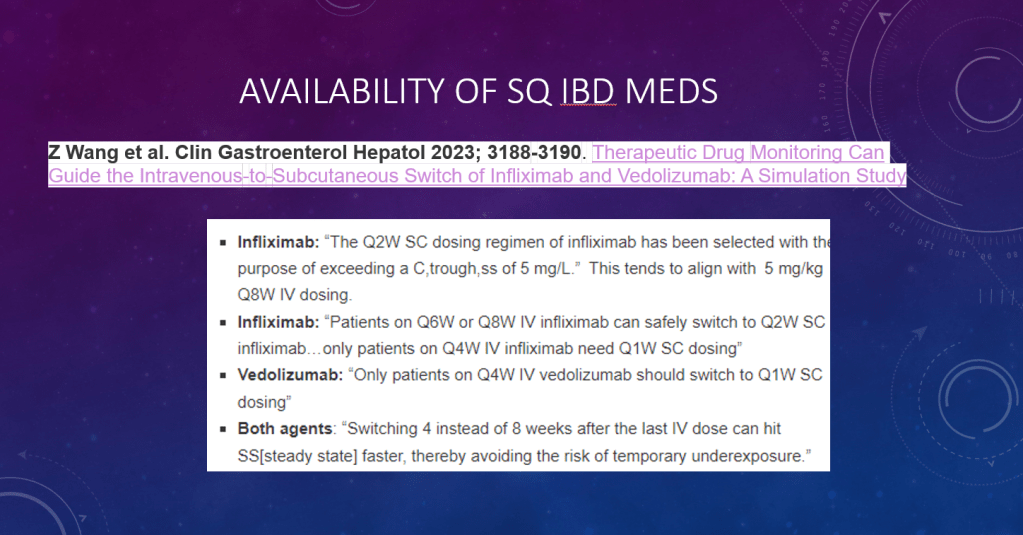
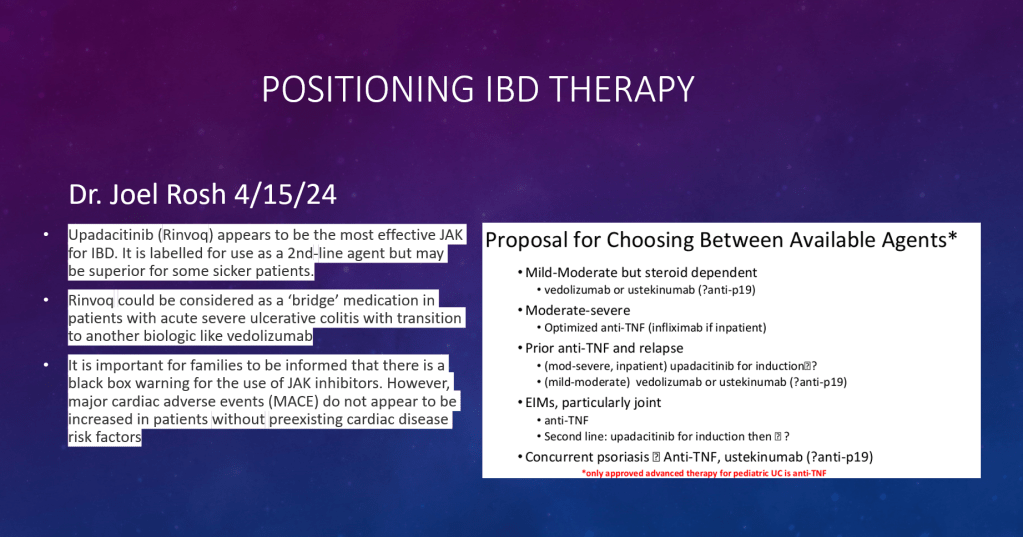
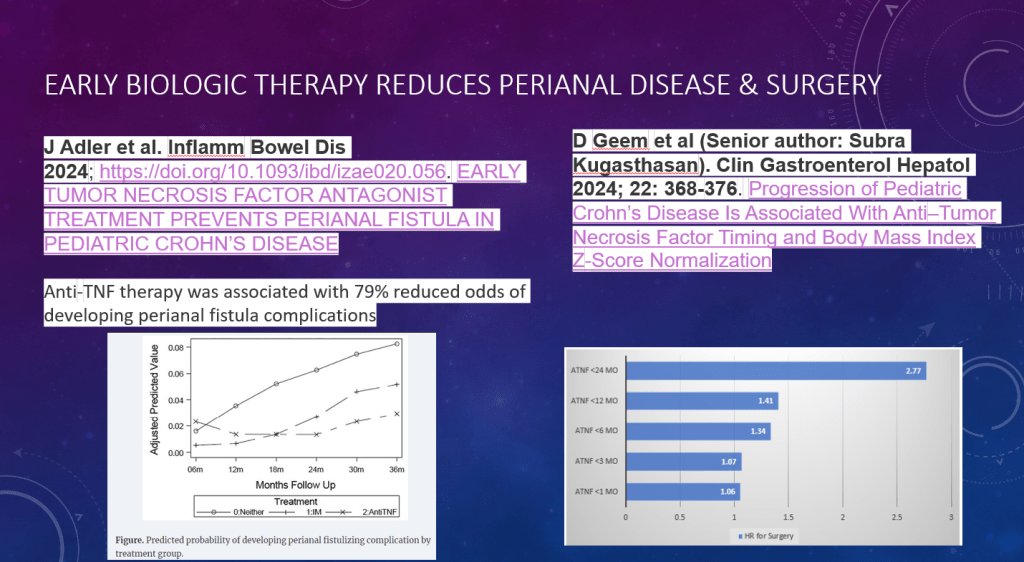
PF van Rheenen et al. JPGN 2024; DOI: 10.1002/jpn3.12378. Open Access! Primary sclerosing cholangitis in children with inflammatory bowel disease: An ESPGHAN position paper from the Hepatology Committee and the IBD Porto group
Recommendations:
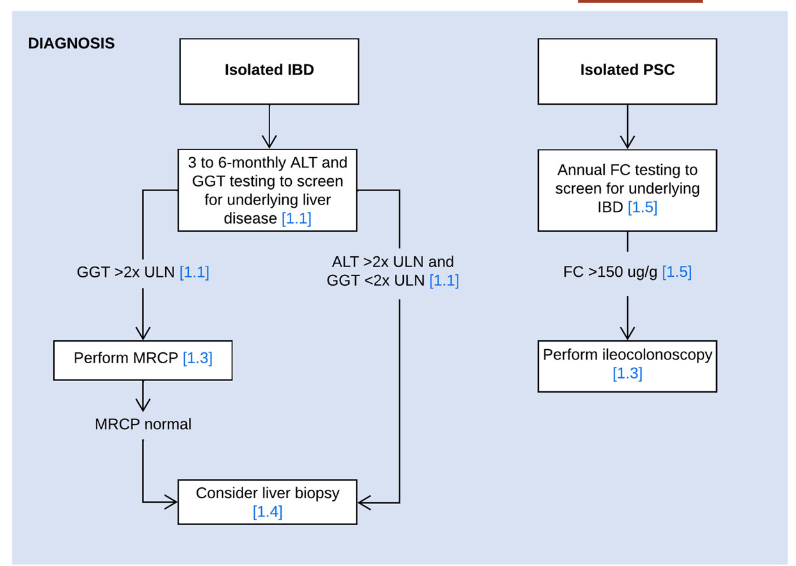
- In children with suspected or confirmed IBD, screening for liver disease is usually performed at 3 to 6 months intervals and a work‐up for underlying liver disease is most commonly initiated when liver enzymes exceed 2x the upper limit of normal
- Use MRCP as the radiological modality of choice for diagnosing PSC
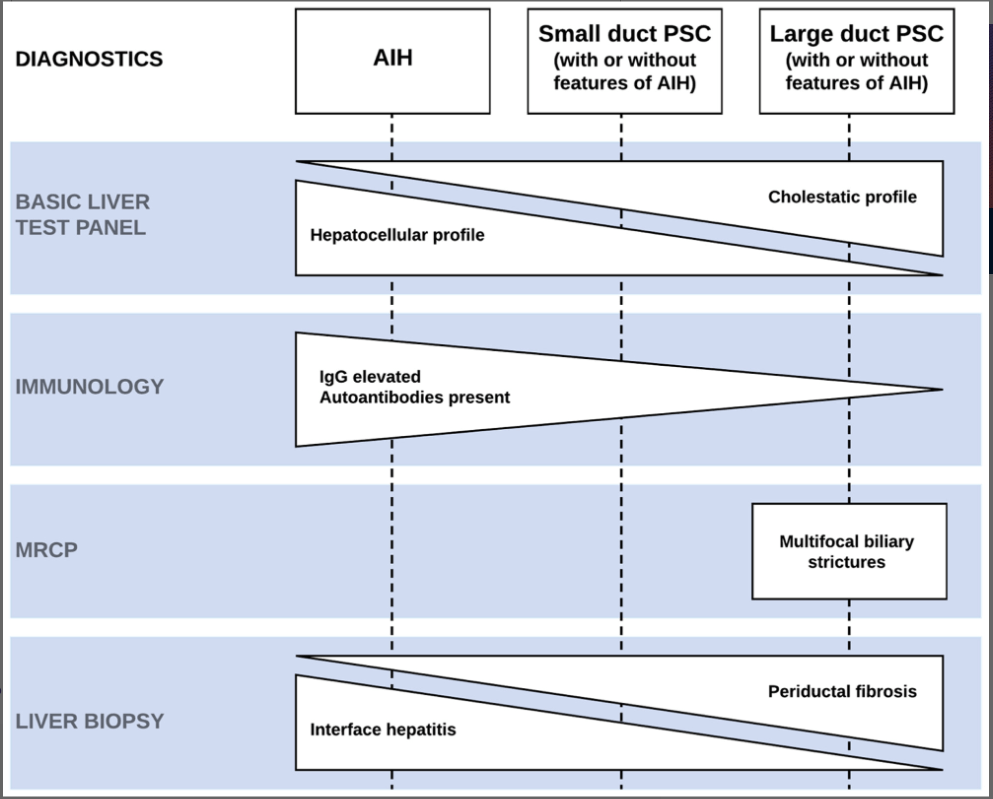
- Consider performing a liver biopsy in children with IBD and suspected PSC in the following circumstances: i) Normal biliary tree at MRCP, ii) raised immunoglobulin G and the presence of liver-specific autoantibodies, or iii) clinical uncertainty before steroid induction therapy for IBD
- Perform fecal calprotectin screening at least once yearly in children with isolated PSC and/or AIH to select patients for diagnostic endoscopy for suspected inflammatory bowel disease (panel recommends cutoff of >150 indicating need for ileocolonoscopy)
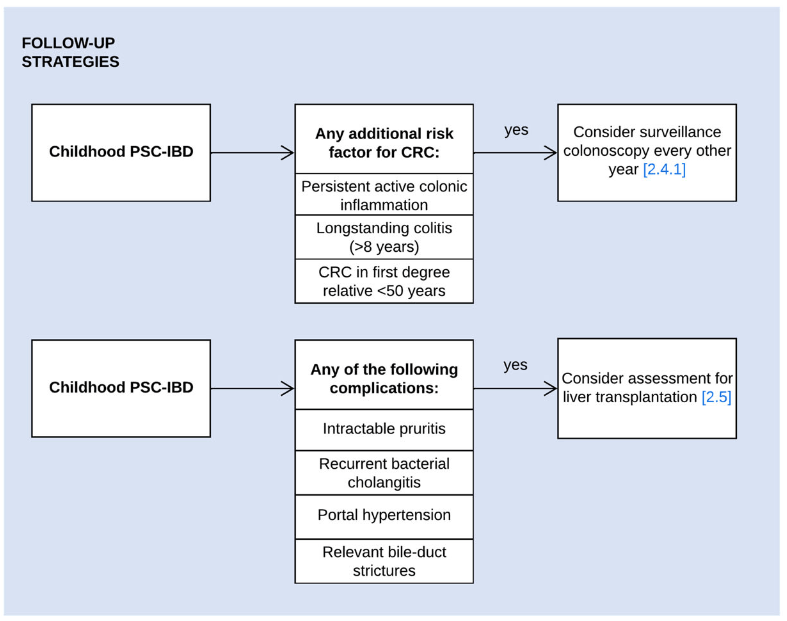
- Surveillance colonoscopy should be considered in children with PSC–IBD and the following risk factors of colorectal cancer: i) persistent active colonic inflammation, ii) longstanding colitis (≥8 years), or iii) a family history of colorectal cancer in a first-degree relative <50 years. (The overall risk of colon cancer in those <18 yrs of age is very low)
- UDCA may be prescribed at doses of 15–20 mg/kg/day. Despite evidence of improvement of liver enzymes, its long-term effect on disease progression has not been demonstrated. Consider a 6-months therapeutic trial of UDCA, either immediately after PSC diagnosis or when spontaneous normalization of GGT does not occur in the first 6 months postdiagnosis. Continue UDCA treatment if there is a meaningful reduction or normalization of GGT or improvement of symptoms
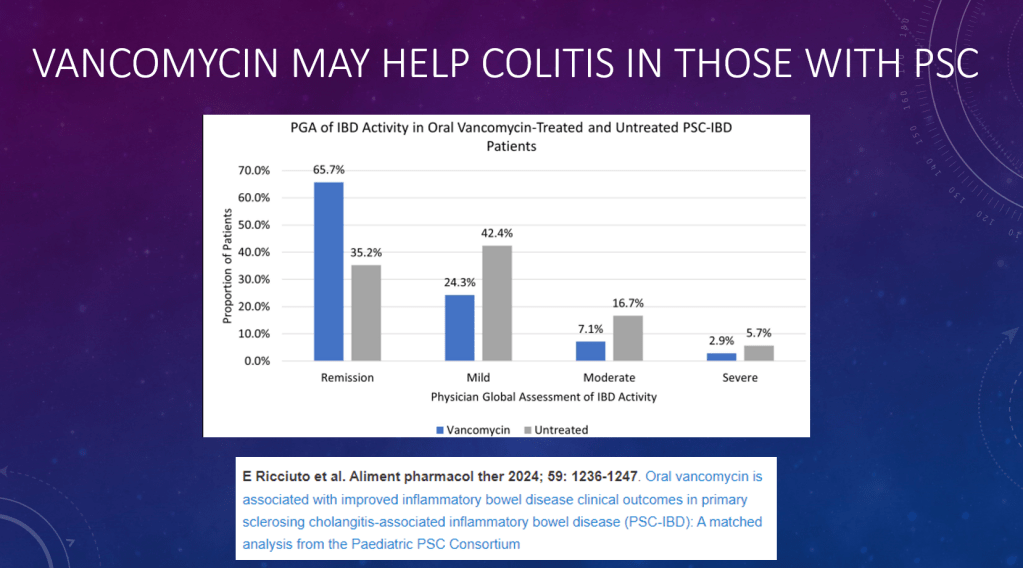
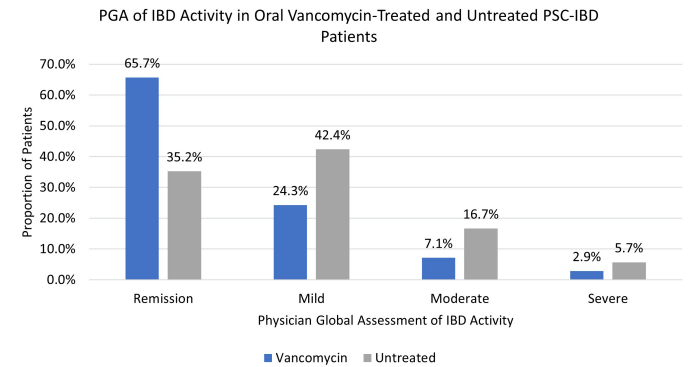
- Oral vancomycin may be prescribed for a potential improvement in liver biochemistry as well as bowel inflammation. Its long-term effect on disease progression has not been demonstrated
- In children with PSC–IBD and biochemical, serological, and histological features of AIH, the use of corticosteroids and antimetabolites may suppress immune-mediated hepatitis. In the absence of convincing AIH features, the use of corticosteroids and antimetabolites is not indicated to manage PSC
- Children with PSC, relevant bile-duct strictures and cholestatic symptoms should be assessed for liver transplantation. When their symptoms are likely to improve following biliary intervention, ERCP can be considered
- Recommended blood testing for children with PSC: At diagnosis: Autoantibodies (ANA, anti-SMA, anti-LKM-1, anti-LC1, and anti-SLA), Every 3-6 months: ALT, AST, GGT, Albumin, INR, Platelets, CRP. Every 12 months: IgG, AFP, and Fat Soluble vitamins. Consider f/u autoantibodies in those with elevated IgG at f/u lab testing
My take: This is a useful position paper; it does not have a zillion recommendations like some other ESPGHAN positions papers. Given the frequency of liver enzyme elevation in patients with IBD, mild to modest elevations may need to be observed before launching an extensive evaluation (see related blog posts below).
Related blog posts:
- Understanding the Reasons for Abnormal Liver Enzymes in Pediatric Inflammatory Bowel Disease This retrospective study provides reassurance that liver enzyme elevations are common in children with IBD, occurring in >40% of patients over 3 years at this center; most often these elevations are benign and transient.
- Vancomycin for Inflammatory Bowel Disease in Patients with Primary Sclerosing Cholantgitis
- PSC in IBD
- Recurrent PSC in Children After Liver Transplantation
- Aspen Webinar 2021 Part 5 -Autoimmune Liver Disease & PSC 2021. This lecture highlights studies show lack of efficacy with vancomycin, ursodeoxycholic acid and vedolizumab in altering the liver disease. Also, there is potential utility of MMP-7 for distinguishing between PSC and AIH
- Liver Problems with Inflammatory Bowel Disease
- Active Colitis More Likely in Children with PSC-IBD
- Big Study of PSC in ChildrenPSC -Natural History Study (pediatric)
- Should We Care About Subclinical Primary Sclerosing Cholangitis with Inflammatory Bowel Disease?
- Primary Sclerosing Cholangitis (PSC) –Natural History Study