RS Dalal et al. Clin Gastroenterol Hepatol 2026; 24: 255-257. One-Year Comparative Effectiveness and Safety of Upadacitinib vs Risankizumab for Crohn’s Disease
This was a retrospective single-center study (n=219) assessing upadacitinib (n=67) or risankizumab (n=152) for active Crohn’s disease (CD). Treatment initiation as post-operative prevention or for non-CD indication were excluded.
**The patients receiving upadacitinib were generally younger, had more anti-TNF/ustekinumab failures, higher CRPs, and higher HBSs compared to risankizumab-treated patients.
Key findings:
- After inverse probability of treatment-weighted (IPTW) analysis, most outcomes were similar between groups. However, upadacitinib-treated patients had more surgeries, adverse events, and treatment discontinuation.

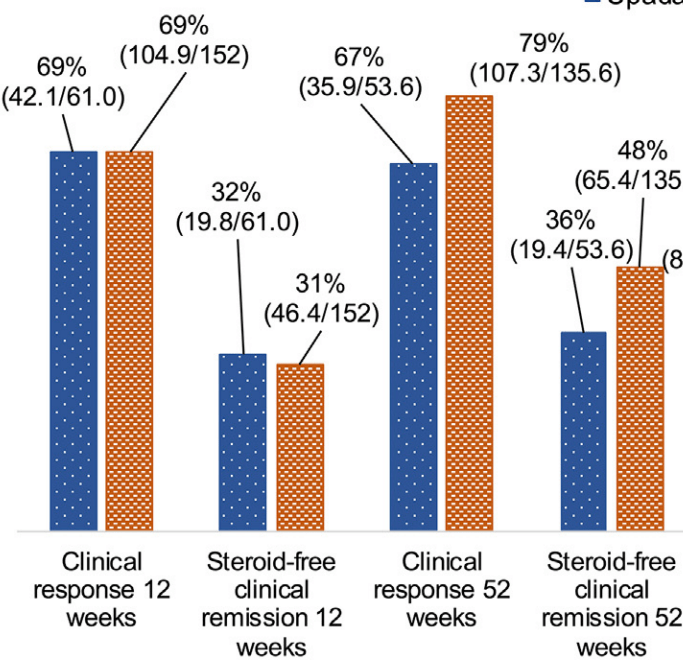
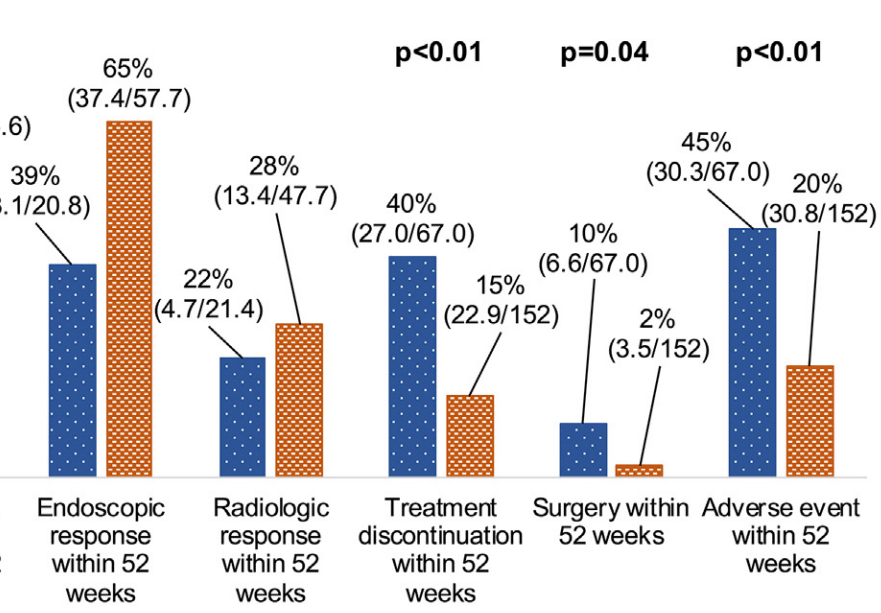
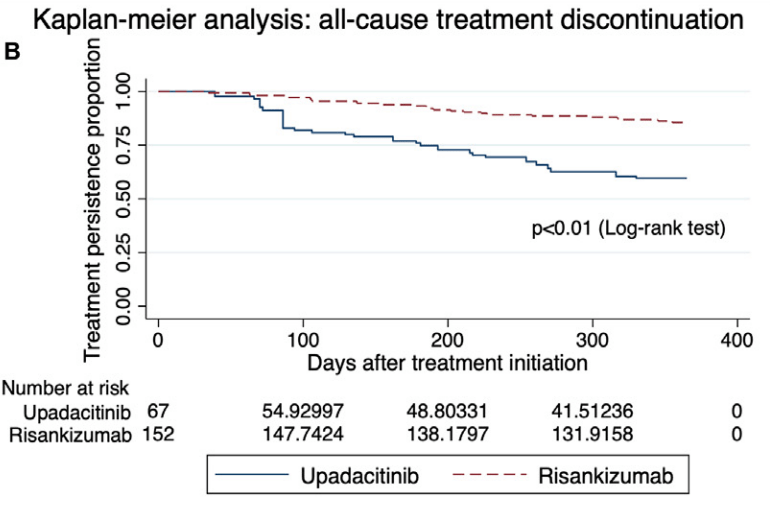
My take: While this study favors risankizumab over upadacitinib, most of the outcomes were fairly similar. Risankizumab may have better long-term durability. However, the observational design limits the conclusions, particularly as the upadactinib-treated patients appeared to be more refractory at baseline. A prospective head-to-head study would be more definitive.
Related blog posts:
- Dr. Maria Oliva-Hemker: Positioning Therapies for Pediatric Crohn’s Disease (2025)
- Spotlight: AGA Living Clinical Practice Guideline on the Pharmacologic Management of Moderate-to-Severe Crohn’s Disease
- AGA Living Clinical Practice Guideline on the Pharmacologic Management of Moderate-to-Severe Crohn’s Disease
- Comparative Evidence and Positioning Advance Therapies for Inflammatory Bowel Disease
