HY Chu et al. NEJM 2025; 398: 817-822. The Path Forward for Vaccine Policy in the United States
This commentary was published on 7/30/25 and was written by the 17 voting members of the ACIP who were dismissed.
Key points:
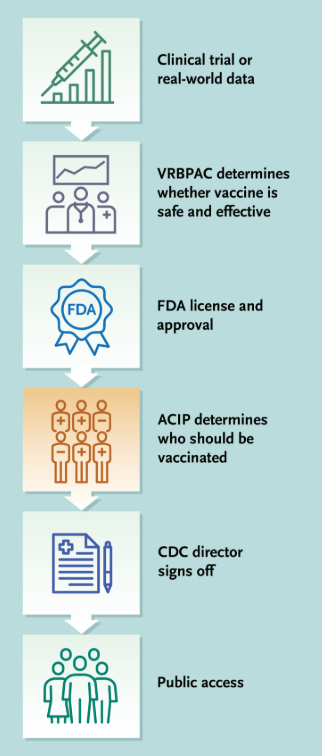
- For over 60 years, the Advisory Committee on Immunization Practices (ACIP), which comprised a diverse group of nonpartisan specialists, has advised the Centers for Disease Control and Prevention (CDC) on vaccine recommendations based on science and intensive review of evidence. The abrupt dismantling of the rigorously vetted process and the replacement of the Committee with an inexperienced and biased panel has engendered fundamental distrust in the Committee’s vital work…The government has abruptly changed vaccine policy through social media postings and publications in news media.
- The ACIP has been an independent committee of vaccine scientists and clinicians that has relied on a process called the Evidence to Recommendations framework. This deliberative framework calls for a review of the strength of evidence around a variety of factors, including the magnitude of the public health problem, potential benefits and harms, values, acceptability, resource use, equity, and feasibility.
- Previously, the ACIP had well-defined and stringent conflict of interest standards. Voting members had to disclose and actively manage any actual or apparent conflicts of interest before and throughout their tenure… ACIP members disclosed any potential conflicts during each vote and could not vote on issues where they had an ongoing conflict.
- ACIP recommendations have many implications. For example, government-run medical systems such as the Veterans Health Administration may be able to provide only vaccines consistent with ACIP recommendations…For children who are uninsured, underinsured, Medicaid-eligible, American Indian, or Alaska Native, the Vaccines for Children program pays only for ACIP-recommended vaccines; about half of children in the United States get their vaccines through this program.
- The nation now faces a scenario in which the rigor and discipline of these vaccine recommendation processes are rapidly eroding…Three major issues are of particular concern: the quality and availability of data; straightforward guidance for providers and the public; and insurance coverage and vaccine access, uptake, and equity.
- The absence of a cohesive federal policy produced by means of an evidence-based, expert-informed process creates the very real potential for conflicting messaging from within the Department of Health and Human Services (DHHS) or in relation to messages from nongovernmental agencies, such as professional organizations. This lack of coordination is likely to cause confusion for providers and the public, vaccine-administration errors, decreased uptake of vaccines, and further erosion of an already damaged public trust. It is also likely that in this milieu, misinformation will flourish.
- The ACIP cannot be replaced, but it may be possible to limit the damage. In this vacuum, it is urgent that other organizations step forward to reassert an evidence-based, expert approach to vaccine recommendations to bring the nation back from the precipice of uncontrolled spread of infectious diseases and needless deaths.
My take: The advice from governmental agencies has been compromised. With regard to vaccines, instead of a transparent process with expertise, we are left with partisan recommendations with questionable credibility.
Related blog posts:
- Warnings of Hepatitis B Vaccine Policy Shift
- Warnings of Hepatitis B Vaccine Policy Shift
- RFK Jr. Ousts Entire CDC Vaccine Advisory Committee
- Rising Scientific Fraud: Threats to Research Integrity Plus One
- ‘What Happens When the Doctors Can’t Trust the Government?’
- “How to Make America Healthy: the Real Problems — and Best Fixes”
- Calamitous Impact of U.S. Withdrawal from Gavi Funding