M Matar et al. J Pediatr Gastroenterol Nutr. 2026;82:487–494. Chronic nonbacterial osteomyelitis associated with pediatric inflammatory bowel disease: : A multicenter retrospective study from the Paediatric inflammatory bowel disease Porto Group of ESPGHAN
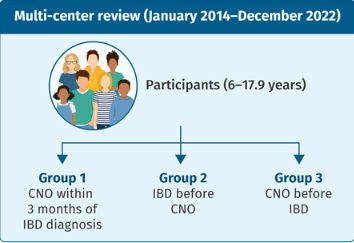
Methods: Retrospective study with 45 pediatric patients with inflammatory bowel disease (n=32 with Crohn’s disease, n=8 with ulcerative colitis, n=5 with IBD-U)
Key findings:
- CNO presented in 15 patients (33%) within 3 months of IBD diagnosis, and in additional 20 (44%) patients after IBD diagnosis; in 10 (22%) patients CNO preceded the diagnosis of IBD with a median time 46 (25–248) weeks
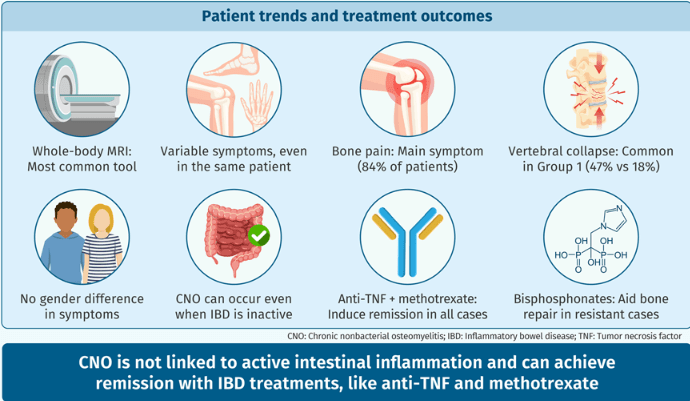
- 11 (24%) subjects displayed at least one additional extra-intestinal manifestations, including arthritis (6, 13%), erythema nodosum (4, 9%), sacro-ileitis (2, 4%), psoriasis (1, 2%), and pyoderma gangrenosum (1, 2%).
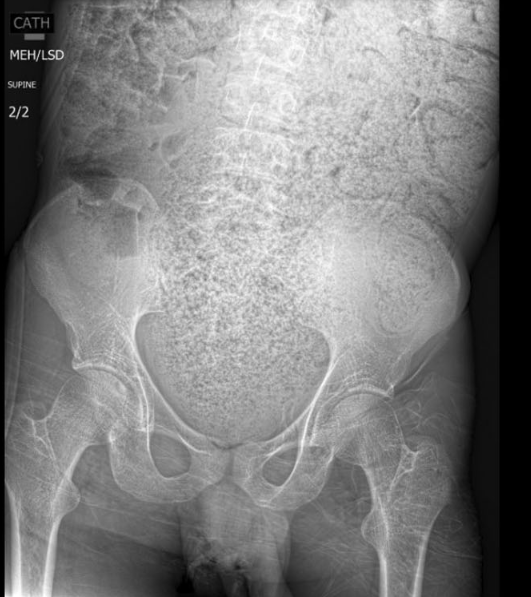
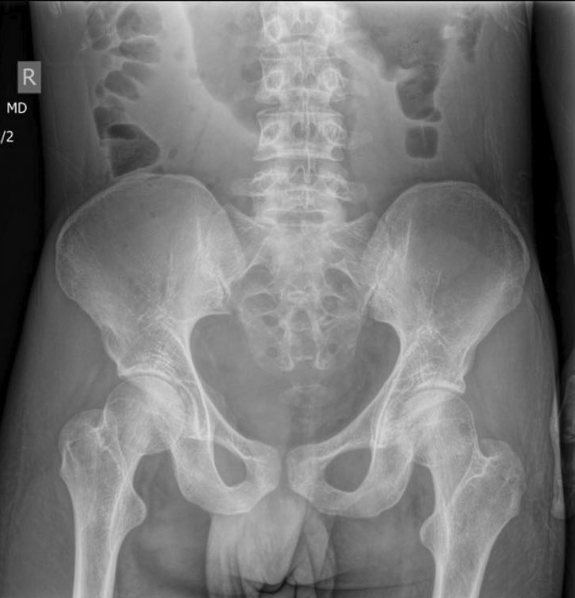
- Complications occurred in six patients and included vertebral collapse, bone fracture, and bone deformity. In eight (18%) subjects vertebral collapse was present already at the time of diagnosis.
- “While in most patients, diagnosis of CNO was associated with either clinical or laboratory indices of active IBD, especially CD, in some cases, the intestinal disease was quiescent.”
- “All patients achieved remission of CNO at some point during follow-up, which may support the hypothesis that anti-TNF treatment is unlikely to promote CNO development and does not reinforce the theory of a paradoxical effect that has been suggested by Cordesse et al.22“
My take: This is a useful review highlighting the association of CNO with both active disease and quiescent IBD. The authors argue that their data does not support the development of CNO as a paradoxical effect. I disagree with this premise. Many of the extraintestinal manifestations, that anti-TNF agents can treat, rarely can be caused by them. Besides CNO, paradoxical reactions to anti-TNF agents have included the development of rheumatoid arthritis, psoriasis, hidradenitis suppurativa and autoimmune liver disease.
Related blog posts:
- Chronic Nonbacterial Osteomyelitis (CNO): What a GI Doctor Should Know
- Paradoxical Immune Mediated Disorders Associated with TNF Inhibitors
- Psoriasis Due to Infliximab –Latest Data
- IBD and Chronic Recurrent Multifocal Osteomyelitis: Paradoxical Association with anti-TNF Therapy in Some Cases
- Pattern of Skin Reactions to Anti-TNF Agents
- Characteristics of Skin Lesions Associated with Anti-Tumor Necrosis Factor Therapy | gutsandgrowth
- Paradoxical Chronic Recurrent Multifocal Osteomyelitis (CRMO)
- Paradoxical Immune Mediated Disorders Associated with TNF Inhibitors
- More on Hidradenitis Suppurativa and Inflammatory Bowel Disease