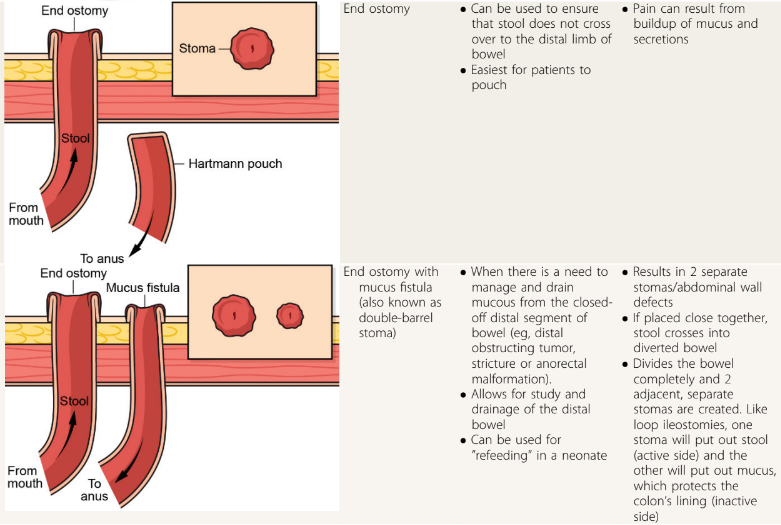
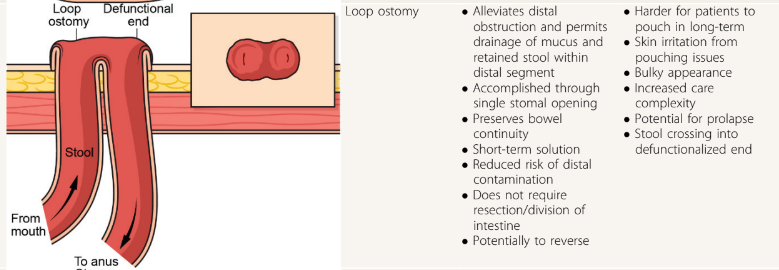
RS Dalal et al. Clin Gastroenterol Hepatol 2024; 22: 666-668. Comparative Effectiveness of Upadacitinib vs Ustekinumab for Ulcerative Colitis: A Multicenter Retrospective Cohort Study
This was a multicenter retrospective cohort study of adults with ulcerative colitis comparing upadacitinib (n=70) to ustekinumab (n=148). The upadacitinib-treated patients were all bio-exposed, had more advanced therapy failures, and higher baseline SCCAI (simple clinical colitis activity index).
Key findings:
- Upadacitinib-treated patients had better outcomes: Clinical response of 82.9% vs. 63.5%, Steroid-free clinical remission 62.1 % vs. 34.7%, improvement in arthralgia 64.3% (9 of 14) vs 23.4% (11 of 47), and endoscopic remission 37.5% (9 of 24) compared with 15.9% (7 of 44).
- The odds ratio (OR) after inverse probability of treatment-weighting were in favor of upadacitinib: Clinical response OR 2.39, SFCR OR 3.17, and endoscopic remission OR 5.10
- Similar amounts of adverse effects were reported in each group
My take: Upadacitinib had better response rates within 52 weeks even though the patients receiving this medication had more advanced therapy failures. However, it is important to keep in mind the limitations of this retrospective study. The improved outcomes are in contrast to a study comparing another JAK inhibitor (tofacitinib) to ustekinumab in which the outcomes appeared equivalent (Tofacitinib vs Ustekinumab -Which is Better for Ulcerative Colitis?).
Related blog posts:
- Upadacitinib Works Quickly and with High Response
- Landmark Study: Oral Biologic for Crohn’s –Upadacitinib
- More Data: Upadacitinib “is Effective and Safe” Plus 2 in Kids
- Bridge Therapy for Ustekinumab with Acute Severe Ulcerative Colitis
- IBD Brief Updates: Anti-TNF Loss of Response, Upadacitinib for ASUC, Risk Factors for Developing IBD
- Ustekinumab for Refractory Pediatric Ulcerative Colitis and Updated Adalimumab Dosing
- Upadacitinib Receives FDA Approval to Treat Adults with Ulcerative Colitis
- New FDA Rinvoq (upadacitinib) Indication: Oral Treatment For Crohn’s
- Tofacitinib vs Ustekinumab -Which is Better for Ulcerative Colitis?