R Loomba et al. NEJM 2023; DOI: 10.1056/NEJMoa2304286. Randomized, Controlled Trial of the FGF21 Analogue Pegozafermin in NASH
Background: Pegozafermin is a long-acting glycopegylated (pegylated with the use of site-specific glycosyltransferases) fibroblast growth factor 21 (FGF21) analogue in development for the treatment of nonalcoholic steatohepatitis (NASH) and severe hypertriglyceridemia
Methods: In this phase 2b, multicenter, double-blind, 24-week, randomized, placebo-controlled trial, 222 patients with biopsy-confirmed NASH and stage F2 or F3 (moderate or severe) fibrosis were randomly assigned patients to receive subcutaneous pegozafermin at a dose of 15 mg or 30 mg weekly or 44 mg once every 2 weeks or placebo weekly or every 2 weeks.
Key findings:
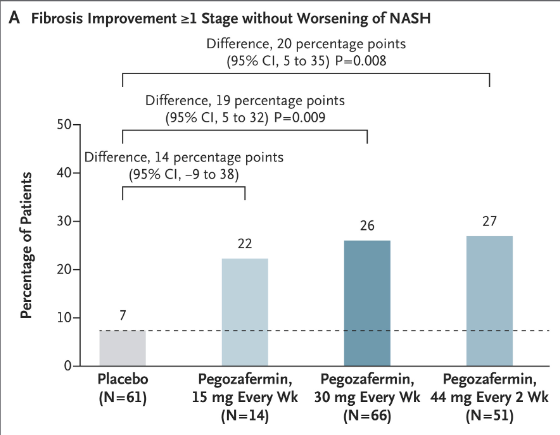
- The percentage of patients who met the criteria for fibrosis improvement was 7% in the pooled placebo group, 22% in the 15-mg pegozafermin group, 26% in the 30-mg pegozafermin group, and 27% in the 44-mg pegozafermin group
- The percentage of patients who met the criteria for NASH resolution was 2% in the placebo group, 37% in the 15-mg pegozafermin group, 23% in the 30-mg pegozafermin group, and 26% in the 44-mg pegozafermin group
- The most common adverse events associated with pegozafermin therapy were nausea and diarrhea.
My take: Thus far, there are no approved pharmacologic therapies for NASH, so this phase 2 study of pegozafermin is an important early step. It is likely that some of the medications which help obesity will likely help with NASH as well.