L D’Antiga et al. NEJM; 2023; 389: 620-631. Gene Therapy in Patients with the Crigler–Najjar Syndrome
Methods: Five patients received a single infusion of the gene construct (GNT0003): two received 2×1012 vector genomes (vg) per kilogram of body weight, and three received 5×1012 vg per kilogram. The primary end points were measures of safety and efficacy; efficacy was defined as a serum bilirubin level of 300 μmol per liter or lower measured at 17 weeks, 1 week after discontinuation of phototherapy. The infusion protocol included administration of sirolimus adjusted for a trough of 4-12 mcg/L (starting 1 week prior to infusion) and steroids (IV day prior then oral for 8 weeks). .
Key findings
- By week 16, serum bilirubin levels in patients who received the lower dose of GNT0003 exceeded 300 μmol per liter.
- The patients who received the higher dose had bilirubin levels below 300 μmol per liter in the absence of phototherapy at the end of follow-up; mean level at the final follow-up visit [week 78 in two patients and week 80 in the other], was149±33 μmol per liter.
- No serious adverse events were reported. Mild increase in ALT levels were seen in 4 of 5 patients; this was “potentially related to an immune response against the infused vector; these patients were treated with a course of glucocorticoids.”
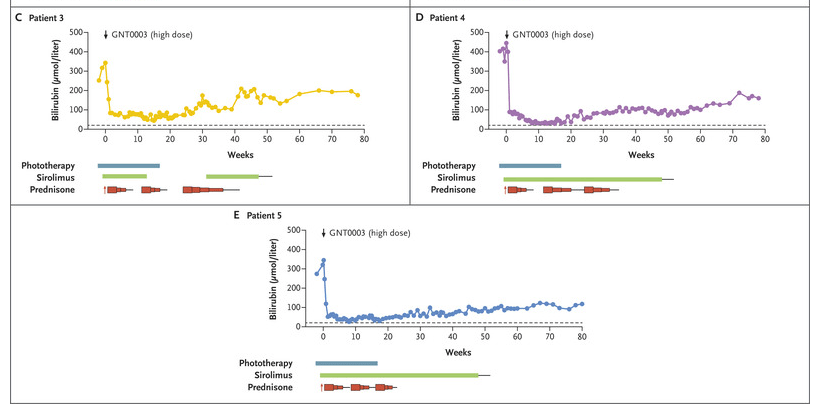
My take: This study shows that the GNT0003 increased UGT1A1 activity to levels that permitted cessation of phototherapy; this persisted for 18 months after treatment. Further studies are needed.
Related blog posts:
