” Takeda (TSE:4502/NYSE:TAK) today announced that the U.S. Food and Drug Administration (FDA) has approved a subcutaneous (SC) administration of ENTYVIO® (vedolizumab) for maintenance therapy in adults with moderately to severely active ulcerative colitis (UC) after induction therapy with ENTYVIO intravenous (IV).1 ENTYVIO SC is expected to be available in the U.S. as a single-dose pre-filled pen (ENTYVIO Pen) by the end of October. Additionally, a Biologics License Application for an investigational SC administration of ENTYVIO for the treatment of adults with moderately to severely active Crohn’s disease is currently under review by the FDA.”
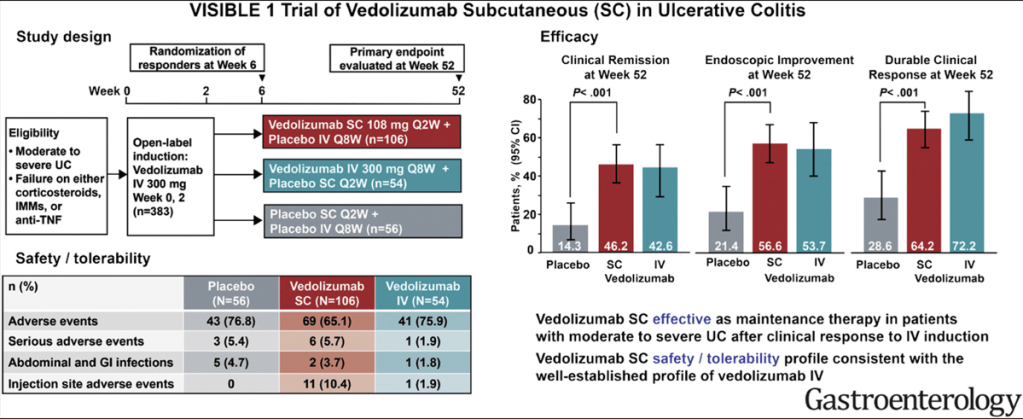
From WJ Sandborn et al. Gastroenterol 2020; 158: 562-572. Open Access! Efficacy and Safety of Vedolizumab Subcutaneous Formulation in a Randomized Trial of Patients With Ulcerative Colitis
In the VISIBLE1 Trial, dosing was IV for week 0 and 2, then every other week SC for maintenance.