C Wester et al. MMWR 2023; 72 (26): 716-720. Open Access! Hepatitis C Virus Clearance Cascade — United States, 2013–2022 (starts on page 16 of PDF)
Key findings:
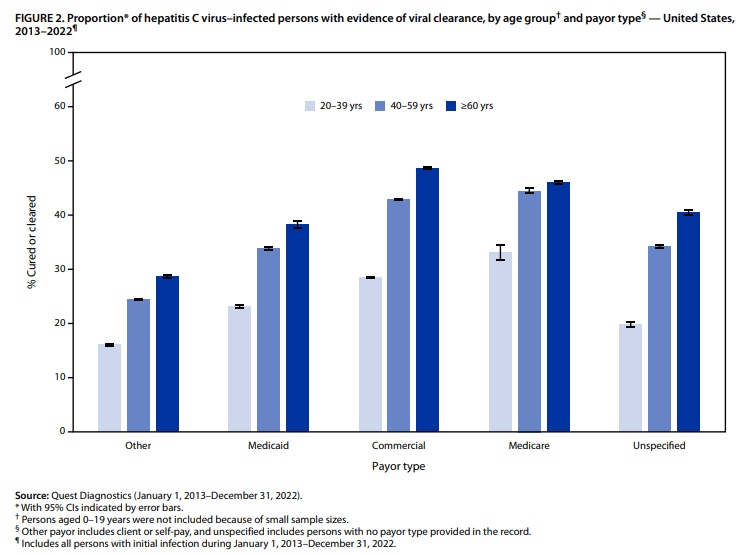
- Among the approximately 1.0 million persons in this analysis with initial infection, only 34% had laboratory evidence of viral clearance
- Overall, viral clearance was lowest among persons aged 20–39 years (24%). Patients 0-19 were not included in this analysis
- To overcome the low cure rate, some have recommended a subscription model for HCV treatment; this was piloted in Louisiana. In this pilot, the state paid a lump sum to make the drug available for free to all patients on Medicaid and federal prisoners. Francis Collins has indicated that a national program, while expensive, would save the government $13 billion in 10 years (Source: Infectious Disease Special Edition, 6/30/23: Most Americans With HCV Not Receiving DAAs)
My take: Improving access to HCV treatment has the potential to save livers, save lives and save money.
Related blog posts:
- Why CDC is Drafting New Guidelines for Screening Children for Perinatally-Acquired Hepatitis C Infection
- NASPGHAN Foundation: Hepatitis C in Children and Adolescents
- History of Medicine: Hepatitis C Discovery –“A Triumph of Curiosity and Persistence”
- Improvement in Hepatitis C Mortality Rates from 2005 to 2017
- The Best Time To Treat Children with Hepatitis C And Cost Considerations
- Hepatitis C in 2020: NASPGHAN Position Paper
