LCMJ Goltstein et al. Gastroenterolog 2024; 166: 690-703. Open Access! Standard of Care Versus Octreotide in Angiodysplasia-Related Bleeding (the OCEAN Study): A Multicenter Randomized Controlled Trial
Methods: The study was designed as a multicenter, open-label, randomized controlled trial. Patients with angiodysplasia bleeding were required to have had at least 4 red blood cell (RBC) units or parental iron infusions, or both, in the year preceding randomization. Patients were allocated (1:1) to 40-mg octreotide long-acting release intramuscular every 28 days or standard of care, including endoscopic therapy.
Key findings:
- Baseline: Patients (n=62, with mean age 72 years) required a mean number of 20.3 transfusion units and 2.4 endoscopic procedures in the year before enrollment.
- During Study: The total number of transfusions was lower with octreotide (11.0) compared with standard of care (21.2). Octreotide reduced the annual volume of endoscopic procedures by 0.9.
- Adverse events: Octreotide-related AEs were common (65%);however, these AEs were mild and self-limiting nature. They “rarely elicit treatment discontinuation.”
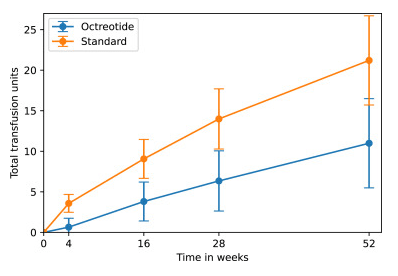
group and standard of care group

My take: Fortunately (for me), angiodysplasia is quite rare in the pediatric age group. In adults, octreotide helps reduce transfusions and need for endoscopy.
Related blog posts:

Pingback: Octreotide in Angiodysplasia-Related Bleeding (the OCEAN Study) – reviewer4you.com