About two years ago, the NEJM published a phase 2 study of Fazisiran for Alpha-One Antitrypsin Deficiency (see: RNA Interference (Fazirsiran) for Liver Disease Associated with Alpha-1-Antitrypsin Deficiency). Now a larger placebo-controlled study which randomized 40 patients to subcutaneous placebo or fazirsiran 25/100/200 mg has been published:
VG Clark et al. Gastroenterol 2024; (in press). DOI:https://doi.org/10.1053/j.gastro.2024.06.028 Fazirsiran for Adults with Alpha-1 Antitrypsin Deficiency Liver Disease: A Phase 2 Placebo Controlled Trial (SEQUOIA)
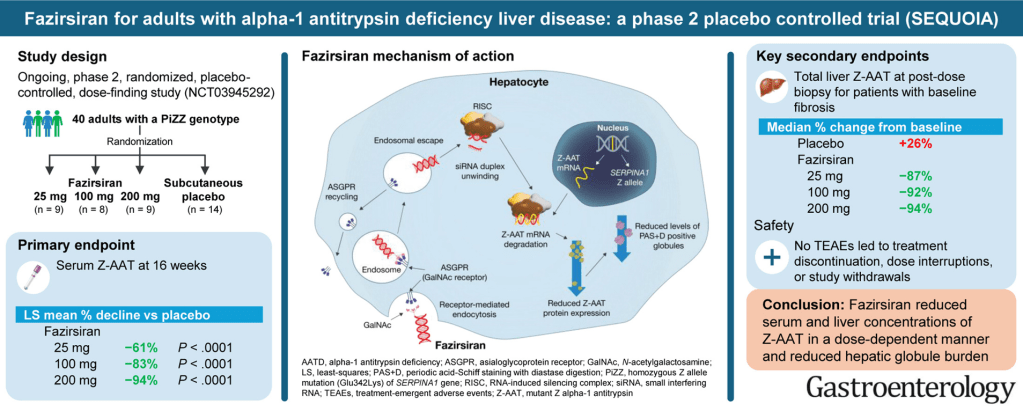
Key findings:
- At Week 16, least-squares mean percent declines in serum Z-AAT concentration were −61%, −83% and −94% with fazirsiran 25/100/200 mg, respectively, versus placebo (all P< .0001)
- Efficacy was sustained through Week 52. At post-dose liver biopsy, fazirsiran reduced median liver Z-AAT concentration by 93% compared with an increase of 26% with placebo
- All fazirsiran-treated patients had histological reduction from baseline in hepatic globule burden
- Portal inflammation improved in 5/12 and 0/8 patients with baseline score >0 in the fazirsiran and placebo groups, respectively
- Histological METAVIR score improved by >1 point in 7/14 and 3/8 patients with fibrosis >F0 at baseline in the fazirsiran and placebo groups, respectively
My take: This is an exciting development for patients with A1AT-associated liver disease. Longer duration data is needed to confirm whether fazirsiran will be a useful therapeutic agent for A1AT deficiency. If effective, selecting patients who benefit from treatment will need to be determined.

Pingback: More Data on Fazisiran for Alpha-One Antitrypsin - reviewer4you.com