AM Jastrebofff et al. NEJM 2025; 392: 958-971. Tirzepatide for Obesity Treatment and Diabetes Prevention
Methods: In this phase 3, double-blind, randomized, controlled trial, there were 2539 participants with obesity, of whom 1032 also had prediabetes. They were assigned in a 1:1:1:1 ratio to receive tirzepatide at a once-weekly dose of 5 mg, 10 mg, or 15 mg or placebo.
Key findings:
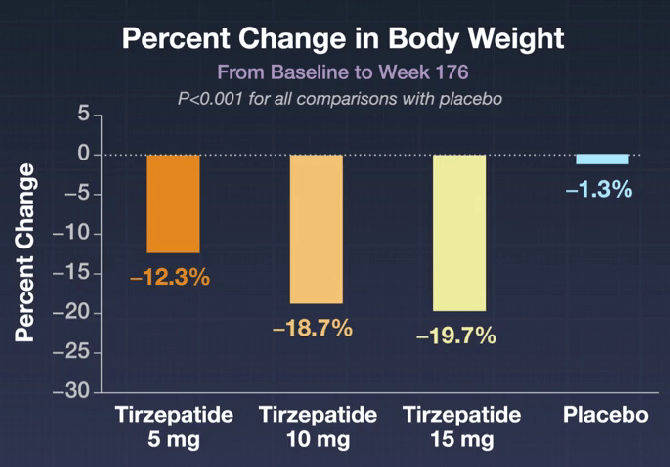
- Weight loss: At 176 weeks, the mean percent change in body weight among the participants who received tirzepatide was −12.3% with the 5-mg dose, −18.7% with the 10-mg dose, and −19.7% with the 15-mg dose, as compared with −1.3% among those who received placebo (P<0.001 for all comparisons with placebo).
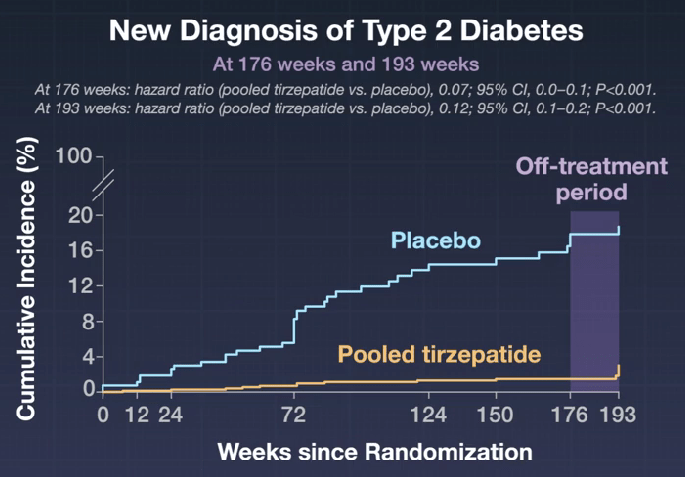
- Type 2 Diabetes Reduction: Fewer participants received a diagnosis of type 2 diabetes in the tirzepatide groups than in the placebo group (1.3% vs. 13.3%; hazard ratio, 0.07). After 17 weeks off treatment or placebo, 2.4% of the participants who received tirzepatide and 13.7% of those who received placebo had type 2 diabetes (hazard ratio, 0.12)
My take: This study shows durable effectiveness of tirzepatide over a three year period with no new safety signals.
Related blog posts:
