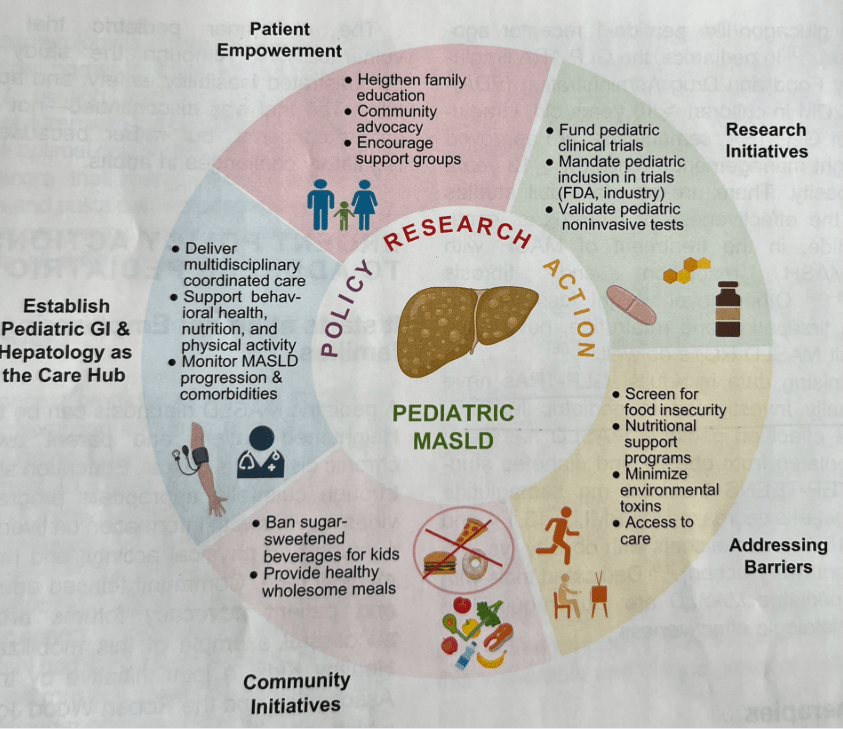
P Hartmann et al. Hepatology 2025; 82: 1341-1351. Call to action—Pediatric MASLD requires immediate attention to curb health crisis
- “Pediatric MASLD is still perceived as an indolent condition by many patients, families, and clinicians. In this Call to Action, we aim to raise awareness of pediatric MASLD as a public health crisis. Herein, we describe insufficient screening and disease staging practices, and a lack of accurate non-invasive tests and effective pharmacotherapy, both stemming from a paucity of multicenter clinical trials in pediatric MASLD.”
- “GLP-1 RAs have not been formally investigated in pediatric MASLD.”
R Schenker et al. JPGN Reports. 2025;1–6. Open Access! Preliminary evidence of improved liver biomarkers in adolescents with obesity and suspected metabolic dysfunction‐associated steatotic liver disease treated with semaglutide: A case series
This was a retrospective study with 5 Latino adolescents obesity and MASLD who received semaglutide for at least 3 months. The range of BMI at the start of treatment was between 51 and 68.
Key findings:
- There were clinically significant reductions in liver enzymes and APRI, a noninvasive marker of fibrosis. Specifically, mean ALT decreased by 38.4 IU/L (p < 0.01), mean AST decreased by 21.0 IU/L (p < 0.01), and mean APRI decreased by 0.128 (p = 0.01)
- All 5 patients experienced weight loss with drop in BMI% from 2.3% to 14.2%
My take: This small study is consistent with others that show GLP1 RAs are likely to be an important tool for patients with MASLD. Current recommendations support use mainly in patients with comorbidities including obesity and T2DM.
Other pediatric studies:
- Choi E, Ramirez Tovar A, He Z, et al. Open Access! Glucagon-like peptide-1 receptor agonists-a potential new medication for pediatric metabolic-dysfunction-associated steatotic liver disease (MASLD). Children (Basel, Switzerland). 2024; 11(3):275
- Children’s Hospital of Philadelphia. Philadelphia TCH of CHOP Study: The Impact of Receptor Agonists on Pediatric MASLD. Children’s Hospital of Philadelphia. This study had 111 patients
Related blog posts:
- AASLD Practice Statement on the evaluation and management of metabolic dysfunction–associated steatotic liver disease in children
- FDA Approves Semaglutide for MASH
- Pharmacological Management of Pediatric Steatotic Liver Disease
- Key Insights on MASLD from Dr. Marialena Mouzaki
- Diets for Obesity and Steatotic Liver Disease Plus Patient Information from FISPGHAN
- Semaglutide’s Efficacy in Phase 3 MASH Trial
- More Data Indicating GLP-1 Efficacy for MASH
- Bariatric Surgery Declines as GLP-1 Medications Rise
- Tirzepatide for Metabolic Dysfunction–Associated Steatohepatitis (MASH) & Uptick in GLP1 Use
- AASLD Practice Changes for Metabolic Liver Disease in 2024
- Bariatric Surgery Declines as GLP-1 Medications Rise
- Survodutide, Dual Glucagon Receptor/GLP-1 Receptor Agonist, for MASH (Phase II Trial)
- Resmetirom (Rezdiffra) -FDA Approved for MASH with Moderate to Advanced Fibrosis
- You No Longer Have Fatty Liver Disease-You Have Steatotic Liver Disease!
- “You Can’t Outrun a Bad Diet”
