AM Tou et al. J Pediatr Gastroenterol Nutr. 2026;82:146–155. Glucagon‐like peptide‐1 receptor agonists in pediatric metabolic dysfunction‐associated steatotic liver disease
Methods: Retrospective study in patients ≤18 years old with a diagnosis of MASLD, who were prescribed a GLP-1RA from January 2, 2018 and January 10, 2024 at the Children’s Hospital of Philadelphia. 42 patients met inclusion criteria (out of a cohort of 111 that had received GLP-1RA with diagnosis of MASLD). Of the GLP-1RA medications, liraglutide was most frequently prescribed (44%), followed by semaglutide (27%), dulaglutide (25%), and exenatide (4%).
Key findings:
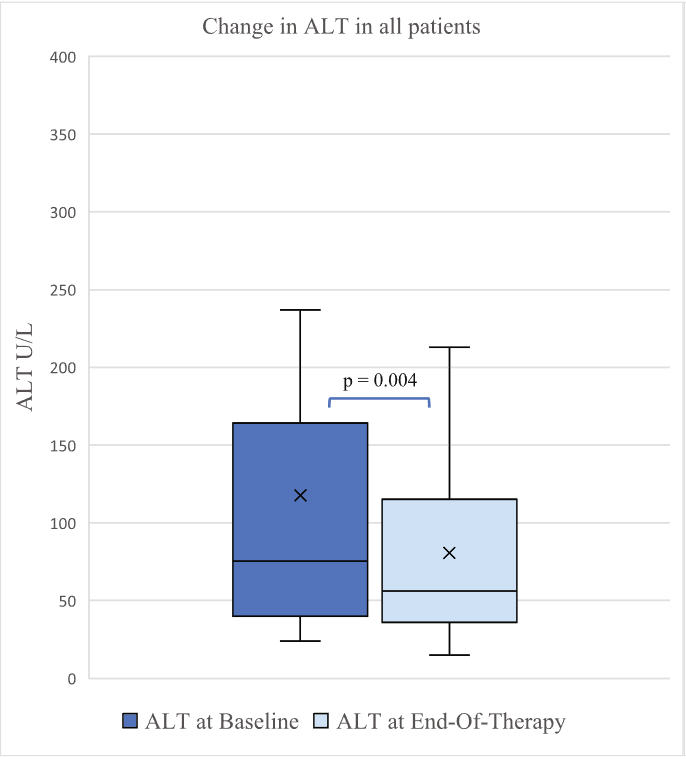
- ALT improved by a mean of 56 U/L at 6 months (p = 0.04), and by 37 U/L at end of treatment (p = 0.004)
- 71% of patients had a therapeutic indication for T2DM and 29% for obesity
- “Improvements were also observed in aspartate aminotransferase (AST), gamma-glutamyl transpeptidase (GGT), glycated hemoglobin (HbA1C), and triglycerides. Body mass index (BMI) percentile and z-score showed no significant changes, but BMI stabilization was observed”
My take: While Semaglutide has an FDA indication for MASLD treatment, there is limited pediatric data. This study indicates that GLP1-RAs are likely to have similar efficacy in adolescents. The lack of weight loss in this study is likely related partly to use in T2DM which has a lower response and the use of GLP-1RAs known to have lower response with regard to weight loss.
Related blog posts:
- AASLD Practice Statement on the evaluation and management of metabolic dysfunction–associated steatotic liver disease in children
- Two Pediatric MASLD Publications: Call for Action and Use of GLP-1 RAs
- FDA Approves Semaglutide for MASH
- Pharmacological Management of Pediatric Steatotic Liver Disease
- Key Insights on MASLD from Dr. Marialena Mouzaki
- Diets for Obesity and Steatotic Liver Disease Plus Patient Information from FISPGHAN
- Semaglutide’s Efficacy in Phase 3 MASH Trial
- More Data Indicating GLP-1 Efficacy for MASH
- Bariatric Surgery Declines as GLP-1 Medications Rise
- Tirzepatide for Metabolic Dysfunction–Associated Steatohepatitis (MASH) & Uptick in GLP1 Use
- AASLD Practice Changes for Metabolic Liver Disease in 2024
- Bariatric Surgery Declines as GLP-1 Medications Rise
- Survodutide, Dual Glucagon Receptor/GLP-1 Receptor Agonist, for MASH (Phase II Trial)
- Resmetirom (Rezdiffra) -FDA Approved for MASH with Moderate to Advanced Fibrosis
- You No Longer Have Fatty Liver Disease-You Have Steatotic Liver Disease!
- “You Can’t Outrun a Bad Diet”
