Dr. Jose Garza joined our group in 2013 and has been providing excellent care for children throughout the South with suspected motility disorders. Recently, he gave our group a fabulous update on what’s new in motility. My notes below may contain errors in transcription and in omission. Along with my notes, I have included many of his slides.
FLIP -How Distensible is the Esophagus
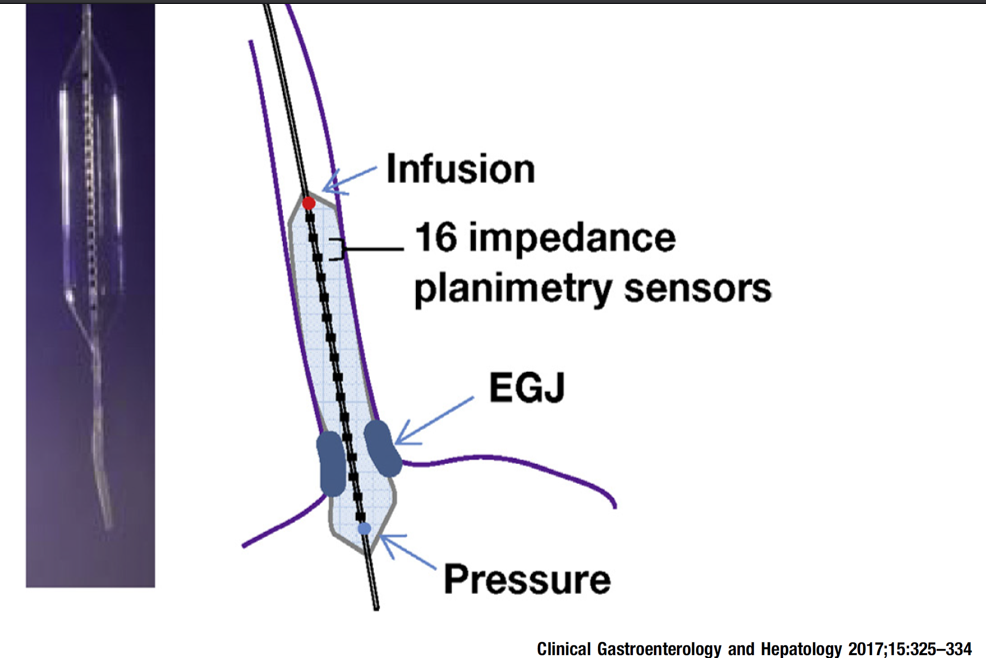
- Distensibility index less than 2 mm2/mmHg is considered abnormal.
- Normal FLIP (presence of RACs and normal Distensibility Index) can obviate need for high resolution esophageal manometry (HREM)
- FLIP during anesthesia can only be done with certain medications: versed, fentanyl and propofol (no gas)
- FLIP is useful in evaluating if symptoms after achalasia treatment, during achalasia treatment (dilatation or Heller), if symptoms after fundoplication, and if manometry uncertain


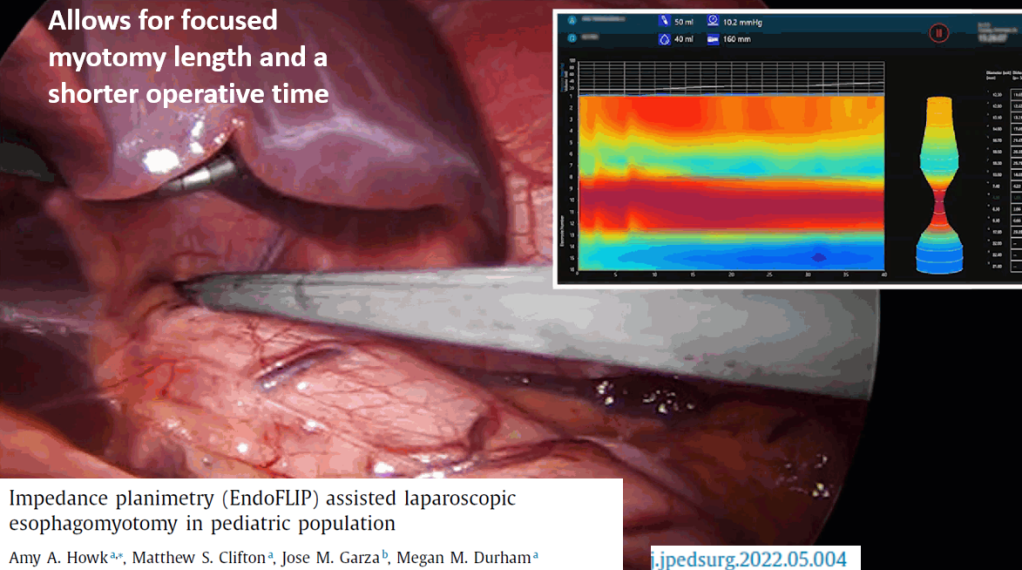
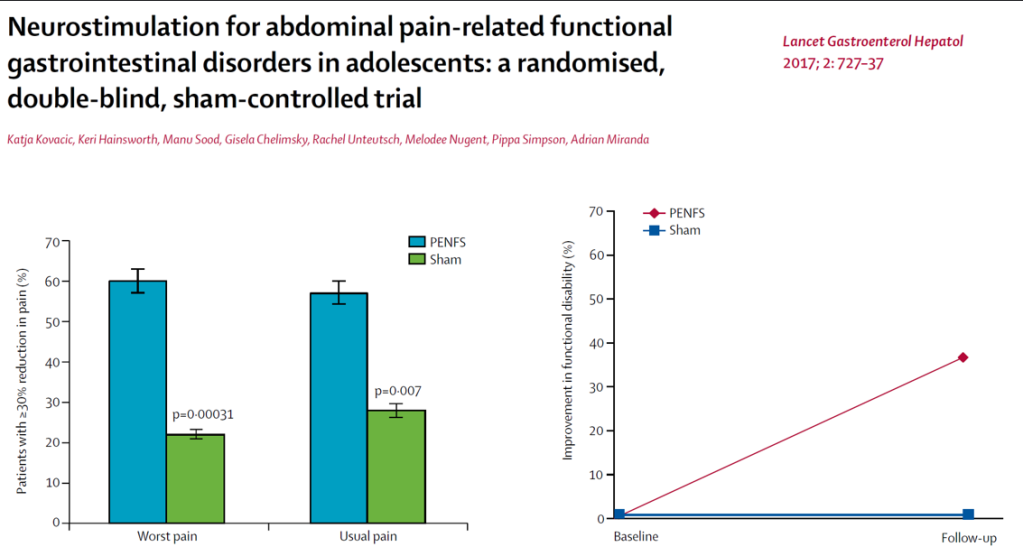
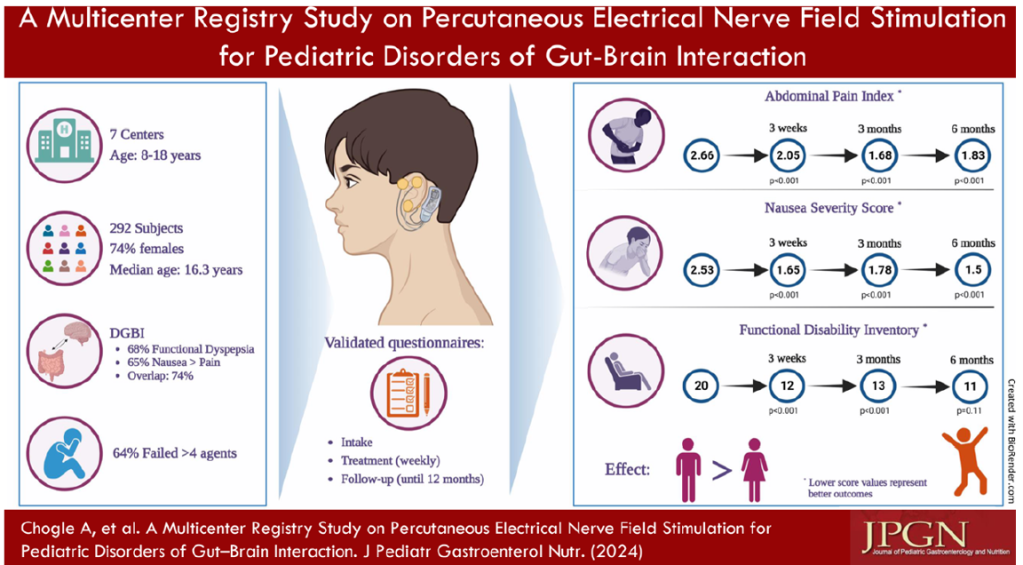
IB-Stim
- Good results in improving pain, sleep, nausea and function
- Could be a 1st line treatment though cost can be an issue
- Related posts: IB-Stim (Neuro-Stim) for Adolescents with Irritable Bowel, Auricular Stimulation Associated with Less Pain, Less Disability, and Better Sleep, Neuro-Stim for Refractory Cyclic Vomiting?

Unable to Belch ( (Retrograde Cricopharyngeus Dysfunction)
- Inability to burp can be due to retrograde cricopharyngeal dysfunction. Symptoms can include chest pain, gurgling noises, bloating
- HREM can show UES not relaxing/air trapping in esophagus after carbonated beverage challenge.
- Treatment: ENT can inject botox to UES. This is usually a one-time therapy which may help stop a learned behavior.
- Related JPGN article: Pediatric retrograde cricopharyngeal dysfunction diagnosed by high-resolution impedance manometry. Dorfman L, El-Chammas K, Mansi S, Graham K, Kaul A.
Sitz Markers
- Guideline published suggest single xray on day 5 (usually off treatment). Cleanout recommended (especially if impacted)
- Useful for non-retentive fecal soiling as well

Vibrating capsule
- Adult study showed efficacy for constipation
- Related post; Price Per Poop with Vibrating Capsule and New AGA Constipation Guidelines

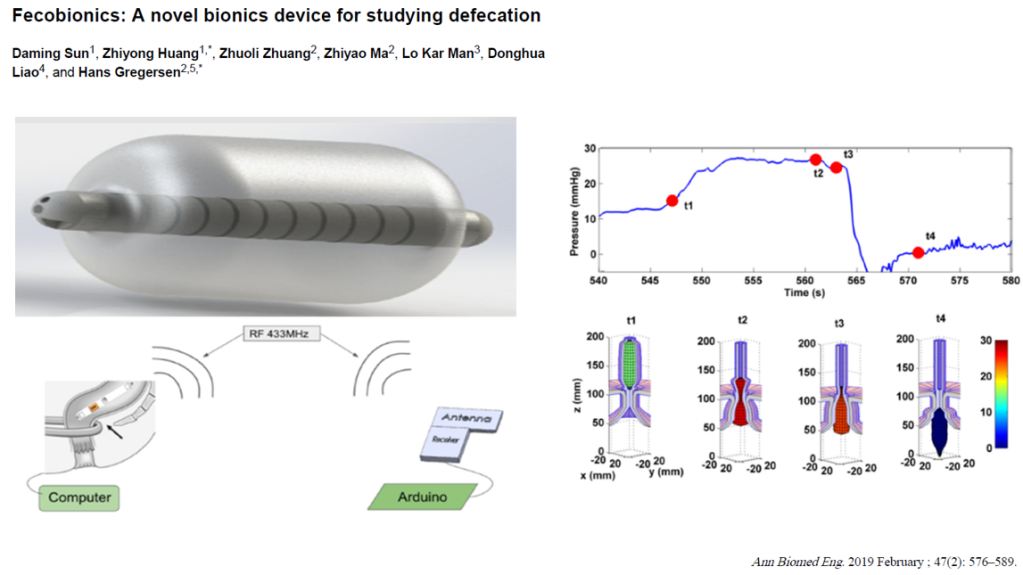
Fecobionic
- Emerging technology to help provide more data on defecation dysfunction
Medications:
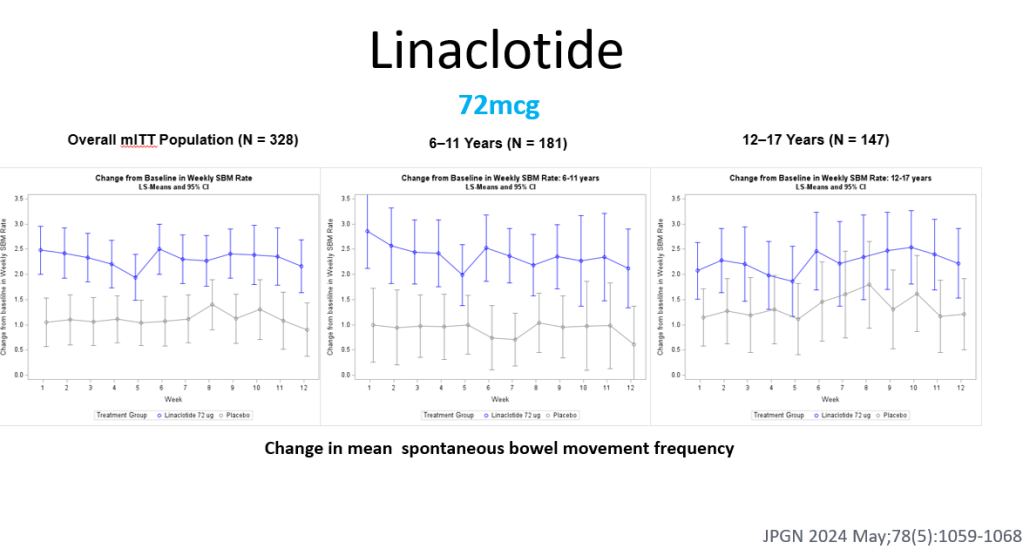
- Linaclotide effective in randomized controlled-trial of children (dose 72 mcg). In this study, 6-11 yr olds had better response and it is possible that a higher dosage would be more effective in adolescents. Safe profile -can cause diarrhea. Related post: Linaclotide -Now FDA-Approved for Children
- Gabapentin shown to have similar effectiveness as baclofen for chronic cough.
- Baclofen is being used more frequently as an adjunct with rumination. Related posts: Most Kids with Rumination Respond to Specialized Treatment, Baclofen for Rumination
- Prucalopride helps with esophageal peristalsis, upper GI symptoms and with constipation. There was a negative pediatric study for treatment of constipation but the study enrolled a lot of younger patients (not likely helpful in overcoming withholding). Insurance coverage often precludes use in younger age group. Related post: Prucalopride -Not Better Than Placebo for Children with Constipation
- Mirtazapine has been helpful for chronic nausea. The typical starting dose in teens 15 mg at bedtime. (related posts: Mirtazapine for Functional Dyspepsia, Dreaded Nausea (2022) Plus Skills or Pills)

Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.

