Last fall, the FDA approved a liquid version of omeprazole.
9/3/22 Drugs.com: “FDA Approves Konvomep. FDA Approves Konvomep (omeprazole and sodium bicarbonate for oral suspension) for Gastric Ulcer and Reduction of Risk of Gastrointestinal Bleeding in Critically Ill Patients
WOBURN, Mass. (September 2, 2022) – Azurity Pharmaceuticals, Inc., a pharmaceutical company focused on developing innovative dose-forms and formulations of products to serve the needs of overlooked patients, announced today that the U.S. Food and Drug Administration (FDA) has approved Konvomep (omeprazole and sodium bicarbonate for oral suspension).“
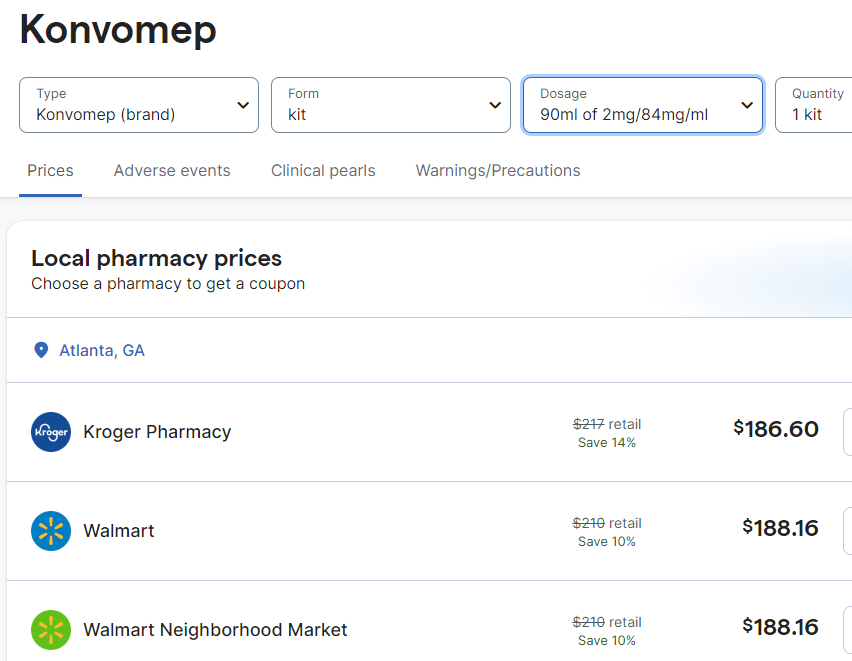
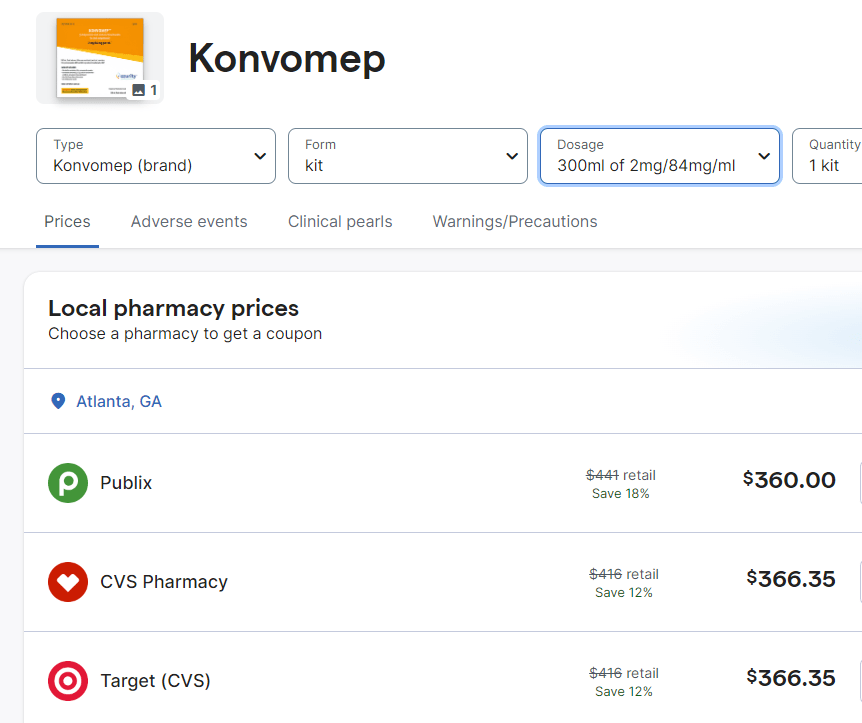
Now that this FDA approved product is available, it may be that it will be more difficult to receive a compound version. The cost of this new formulation is much higher. Here are some of the costs from GoodRx.com.
My take: The high cost of this liquid preparation is another good reason to avoid using a PPI in patients with low likelihood of benefit.
Related blog posts:
- Does PPI Use Increase Pneumonias in Otherwise Healthy Infants?
- Association and Causation: Early Life Risk Factors for Eosinophilic Esophagitis
- No Effect of Proton Pump Inhibitors and Irritability on Crying in Infants | gutsandgrowth
- Long-term effects on bone health due to PPIs given in infancy
- 2018 Pediatric GERD Guidelines
- How Many Kids with Reflux have Reflux?