Home | About Jay Hochman -Pediatric Gastroenterology Blog | Archives
January 26, 2018 7:03 am
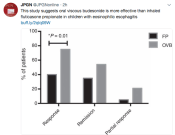
A recent retrospective study (JM Fable et al. JPGN 2018; 66: 26-32) found that patients with eosinophilic esophagitis (EoE) who were treated with oral viscous budesonide (OVB) had more favorable outcomes than those treated with fluticasone propionate (FP). This single center study included 68 pediatric patients (mean age 10.6 years) with 20 receiving FP and 48 OVB.
Dosing in study:
Key findings:
Since this is a retrospective study, there are several potential limitations, including possible selection bias. In addition, higher doses of topical agents have been shown to have higher response rates.
My take: Budesonide is probably better than fluticasone for EoE and its high first-pass metabolism indicates that it is probably safer as well.
Related blog posts:
Posted by gutsandgrowth
Categories: Pediatric Gastroenterology Intestinal Disorder
Tags: eosinophilic esophatitis, fluticasone propionate, oral viscous budesonide
Mobile Site | Full Site
Get a free blog at WordPress.com Theme: WordPress Mobile Edition by Alex King.
1. Proper technique with fluticasone inhaler is very important. In light of retrospective study, unlikely that was accounted for.
–side note: Denver Children’s/Glen Furuta, made a really nice video on proper technique (https://youtu.be/0x7IXhgTsb0)
2. Apologies for not reading entire article, but not sure your comment on bio-availability is correct. I’ve seen data on fluticasone being <1% and budesonide being 10-15%.
–Aliment Pharmacol Ther 1998, 12: 591-603
–J Allergy Clin Immunol, 1998, Vol. 101, Number 4, Part 2: S440-S446
By Benjamin Enav on January 26, 2018 at 9:41 am