DQ Huang et al. Hepatology 2023; 78: 1558-1568. Antiviral therapy substantially reduces HCC risk in patients with chronic hepatitis B infection in the indeterminate phase
Key findings:
- After inverse probability of treatment weighting (IPTW) (n = 819), the 5-, 10-, and 15-year cumulative HCC incidence was 3%, 4%, and 9% among treated patients (n = 394) versus 3%, 15%, and 19%, among untreated patients (n = 425), respectively (p = 0.02)
- It took 5 years of treatment before there was a significant reduction in HCC risk
- The protective effect was mainly in males; it was not observed in females and in patients who were HBeAg negative
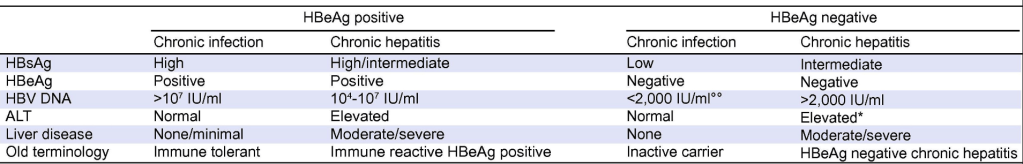
The author studied patients with “Indeterminate” HBV; that is, patents that did not fit into the following categories:


My take: In this subgroup with indeterminate-phase chronic hepatitis B, antiviral treatment resulted in a 70% reduction in HCC risk. Previous AASLD guidelines indicated that treatment is mainly beneficial for immune active HBV; this study indicates that adults with indeterminate-phase HBV benefit as well. Also, as noted in prior blog posts (see below), the term “immune tolerant” is falling out of favor. In addition, updated expert recommendations on expanding treatment have been published: P Martin et al. Clin Gastroenterol Hepatol 2022; 20: 1766-1775 (post: What’s New in the Treatment of Hepatitis B (2022)
Related blog posts:
- Comprehensive 2018 AASLD Guidance for Chronic Hepatitis B
- Why Fewer Children Have Immune-Tolerant Hepatitis B Infection Than Previously
- “Immune-tolerant” — a misnomer for HBV infection