Previous work has established Guselkumab, a IL-23p19 subunit antagonist for Crohn’s disease (Guselkumab: Expanding the GALAXI of Treatments for Crohn’s Disease).
Peyrin-Biroulet et al now provide data showing its efficacy for ulcerative colitis (UC): Gastroenterol 2023; 165: 1443-1457. Open access! Guselkumab in Patients With Moderately to Severely Active Ulcerative Colitis: QUASAR Phase 2b Induction Study
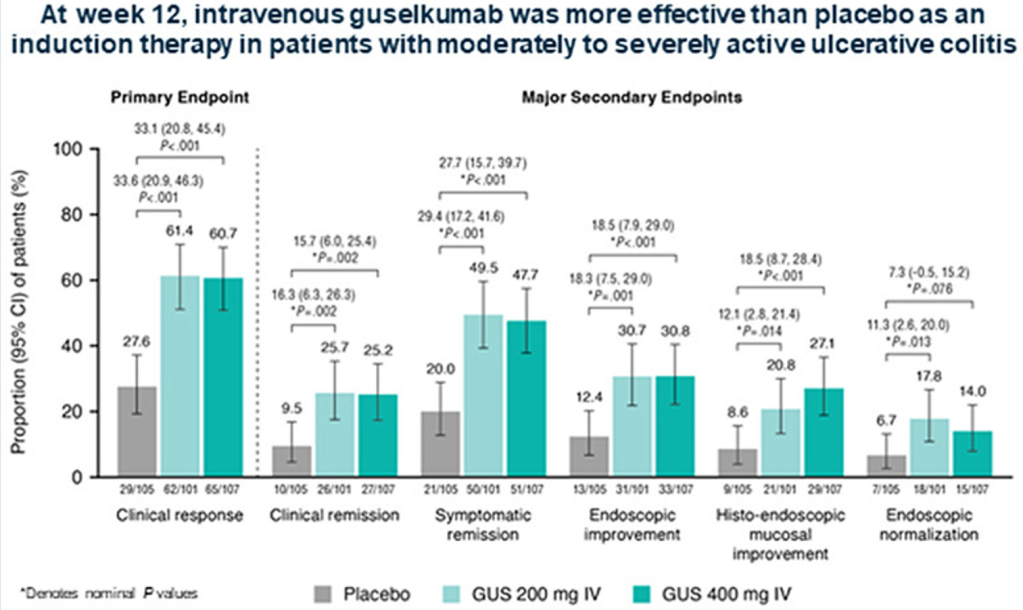
Background/Methods: The QUASAR Phase 2b Induction Study evaluated the efficacy and safety of guselkumab, an interleukin-23p19 subunit antagonist, in patients with moderately to severely active ulcerative colitis (UC) with prior inadequate response and/or intolerance to corticosteroids, immunosuppressants, and/or advanced therapy. In this double-blind, placebo-controlled, dose-ranging, induction study, adult patients (n=313), with median disease duration of 7.5 years, were randomized (1:1:1) to receive intravenous guselkumab 200 or 400 mg or placebo at weeks 0/4/8.
Key findings:
- Week-12 clinical response percentage was greater with guselkumab 200 mg (61.4%) and 400 mg (60.7%) vs placebo (27.6%; both P < .001). (Patients received IV induction at 0,4, and 8 weeks)
- Greater proportions of guselkumab-treated vs placebo-treated patients achieved all major secondary endpoints (clinical remission, symptomatic remission, endoscopic improvement, histo-endoscopic mucosal improvement, and endoscopic normalization) at week 12
- Among guselkumab week-12 clinical nonresponders, 54.3% and 50.0% of patients in the 200- and 400-mg groups, respectively, achieved clinical response at week 24 (after another dose of guselkumab (2nd dose SC). Thus, by week 24, 80.2% (81/101) of patients in the 200 mg IV induction and 78.5% *84/107) in the 400 mg IV induction had a clinical response.
- Clinical response was noted as early as 2 weeks (first timepoint assessed)
- Safety was similar among guselkumab and placebo groups.
My take: This is an era with rapidly expanding medical treatments for inflammatory bowel disease; it should help reduce the problem of individuals who are refractory to available treatments.