Last year, the FDA approved linaclotide, a guanylate cyclase C agonist, for the treatment of pediatric constipation (related post: Linaclotide -Now FDA-Approved for Children).
Here’s the phase III study data: C DiLorenzo et al. The Lancet Gastroenterol Hepatol 2024; 9: 238-250. Efficacy and safety of linaclotide in treating functional constipation in paediatric patients: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial
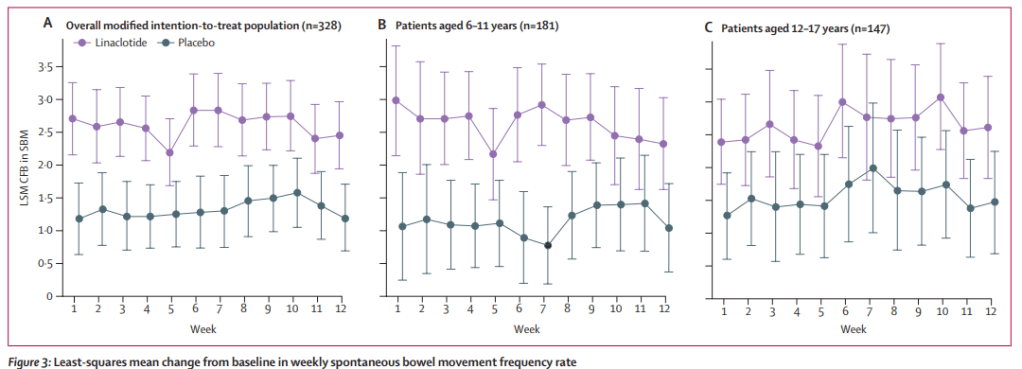
Children 6-17 yrs of age (n=328) received either oral linaclotide 72 μg or placebo once daily for 12 weeks. Key findings:
- The mean frequency rate for spontaneous bowel movements (SBMs) was 1·28 SBMs per week (SD 0·87) for placebo and 1·16 SBMs per week (0·83) for linaclotide, increasing to 2·29 SBMs per week (1·99) for placebo and 3·41 SBMs per week (2·76) for linaclotide during intervention.
- Linaclotide also significantly improved stool consistency over placebo
- Straining with stooling also improved. Change from baseline improvement (Least squares mean change) −1·19 in linaclotide group compared to −0·75 in placebo group (p<0·0001). Straining scale based on responses to the following question: When you pooped, how hard did you push? (0=not hard at all; 1=I pushed a tiny bit hard; 2=I pushed a little hard; 3=I pushed hard; 4=I pushed very hard)
- Abdominal bloating improved as well compared to placebo, Change from baseline improvement (Least squares mean change): −0·51 vs −0·35 (p=0·027)
- The authors note that “the subgroup analysis by age for CFB in SBM frequency rate suggests that the response of the older cohort (aged 12−17 years) was not as strong as the response of the younger cohort (aged 6–11 years).” This could indicate that a higher dose may be beneficial in this age group. “Further studies evaluating increased dosing regimens for older children will be important.”
- The most frequent treatment-related TEAE was diarrhoea (linaclotide: six [4%] patients; placebo: two [1%] patients).
Here’s the phase II study data: C DiLorenzo et al. JPGN 2024; https://doi.org/10.1002/jpn3.12184. Randomized controlled trial of linaclotide in children aged 6−17 years with functional constipation
This was a multicenter, randomized, double-blind, placebo-controlled phase 2 study, with 173 children with functional constipation (based on Rome III criteria) were randomized to once-daily linaclotide or placebo.
Key findings:
- A numerical improvement in mean spontaneous bowel movement (SBM) frequency was observed with increasing linaclotide doses (1.90 in 6- to 11-year-olds [36 or 72 μg] and 2.86 in 12- to 17-year-olds [72 μg]).
- The most reported treatment-emergent AE was diarrhea, with most cases being mild; none were severe. The most reported treatment-emergent AE was diarrhea, with most cases being mild; none were severe.

My take: It is good news to have the an FDA-approved treatment for pediatric functional constipation. It is worth remembering that the estimated cost for a monthly supply is $560 via GoodRx (with coupon, queried on 5/22/24).

Pingback: Linaclotide -Here’s the Pediatric Data - reviewer4you.com