W Afif et al. Am J Gastroenterol; 119: 910-921. Open Access! Efficacy and Safety of Ustekinumab for Ulcerative Colitis Through 4 Years: Final Results of the UNIFI Long-Term Maintenance Study
Background: In the initial UNIFI study, 44% and 38% remission rates were seen after 44 week treatment among patients with and without prior biologic exposure (See post: Ustekinumab for Ulcerative Colitis (UNIFI Trial)).
Methods: Ustekinumab induction responders who completed 44 weeks of maintenance treatment and agreed to enter the long-term extension continued their subcutaneous maintenance therapy (90 mg ustekinumab every 8 or 12 weeks [q8w or q12w] or placebo). Starting at week 56, randomized patients could receive dose adjustment to 90 mg q8w.
Key findings:
- Of the 348 patients randomized to subcutaneous ustekinumab at maintenance baseline (q8w and q12w combined), 55.2% were in symptomatic remission at week 200.
- A greater proportion of biologic-naive patients (67.2% [117/174]) were in symptomatic remission than those with a history of biologic failure (41.6% [67/161]).
- Of the 171 patients with endoscopic evaluation at week 200, 81.6% (71/87) in the q12w group and 79.8% (67/84) in the q8w group had endoscopic improvement.
- Safety: From weeks 156 to the final safety visit (up to week 220), no deaths, major adverse cardiovascular events, or tuberculosis occurred in patients receiving ustekinumab. Nasopharyngitis, UC worsening, and upper respiratory tract infections were the most frequently reported adverse events. “Exposure-adjusted analysis showed that ustekinumab AE rates were not greater than placebo.”
- Immunogenicity: Overall, 5.5% (22/400) of randomized and nonrandomized patients who continued ustekinumab in the LTE were positive for ADA through the final safety visit. Overall, 5 of these 22 patients (22.7%) were positive for neutralizing antibodies. ADA were often transient and seemed to have no effect on efficacy.

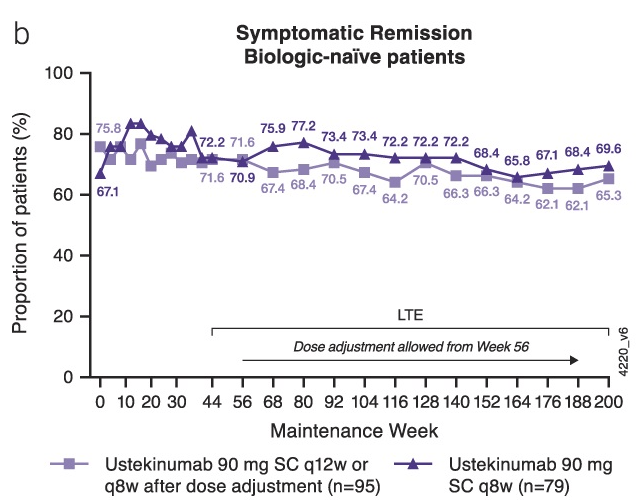

My take: About ~25% of patients starting ustekinumab can expect to be in remission after 4 years based on this study. This estimate is based on a remission rate of ~55% at 200 weeks after achieving clinical remission in ~44% of the initial cohort patients at 44 weeks of treatment. The study provides additional data regarding a favorable safety profile.
Related blog posts:
- Ustekinumab for Ulcerative Colitis (UNIFI Trial)
- Upadacitinib vs Ustekinumab for Ulcerative Colitis
- Comparative Efficacy of Biologics for Crohn’s Disease
- Is Risankizumab More Effective for Crohn’s Disease Than Ustekinumab?
- How Much Ustekinumab (Stelara) Is Needed to Get a Good Response
- Comparative Efficacy: Infliximab vs. Ustekinumab
- Risankizumab Outperforms Ustekinumab (for Crohn’s disease)
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.
