S Ali et al. Clinical Gastroenterology and Hepatology, Volume 22, Issue 10, 2075 – 2083.e1. Characterization of Biologic Discontinuation Among Pediatric Patients With Crohn’s Disease
Methods: Prospective ImproveCareNow registry data (n=823, from 7 centers) were supplemented with medical record abstraction.
Treatment/Monitoring:
- 86% started biologics (78% infliximab, 21% adalimumab, <1% others)
- Twenty-six percent used concomitant immunomodulators for ≥12 months
- Most (85%) measured TDM including 47% induction, 69% proactive, and 24% reactive
Key findings:
- Twenty-nine percent discontinued their first biologic after median 793 days because of inefficacy (34%), anti-drug antibodies (8%), adverse events (8%), or non-adherence (12%)
- Proactive TDM and concomitant immunomodulators were associated with 60% and 32% reduced biologic discontinuation
- Half of patients discontinued biologics without trial of high-dose therapy and 14% without any evaluation
- Among patients started with infliximab therapy, 62% of patients started at a dose of <6 mg/kg, 18% stared at a dose >8 mg/kg. 67% of patients underwent dose escalation. This is agreement with other studies indicating that as many as 80% of children need doses in excess of ‘standard’ dosing (5 mg/kg every 8 weeks)
- In patients with anti-TNF medication inefficacy with TDM availability, 36% had infliximab or adalimumab levels below 5 mcg/mL. and 20% had levels between 6-8 mcg/mL.
- Among patients who discontinued anti-TNF medications, 60% had serum trough levels less than 10 mcg/mL.
- The rate of biologic durability was lower for those (n=61) receiving a 2nd biologic who had rates of remaining on agent of 56% at 1 yr, 28% at 2 yrs, and 10% at 4 yrs. In contrast, the first biologic had durability of 90% at 1 year, 79% at 2 years, and 66% at 4 yrs.
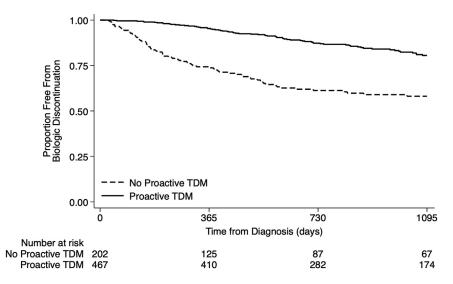
My take: This study strongly supports the use of proactive therapeutic drug monitoring. In addition, the authors make a compelling argument to optimize a therapy and evaluate carefully before switching to a new medication/biologic. Finally, the use of concomitant immunomodulators can improve medication durability; it is particularly important if needing to switch from one anti-TNF agent to another due to anti-drug antibodies.
Related blog posts:
- Proactive Therapeutic Drug Monitoring in Pediatric Crohn’s disease -Better Outcomes (2020)
- Here’s The Proof That Proactive Drug Monitoring Improves Outcomes in Children With Crohn’s Disease (2019). [This blog post summarizes reference #23 in the current study]
- Expert Consensus: New Recommendations for Therapeutic Drug Monitoring (2021)
- Can Therapeutic Drug Monitoring with Monotherapy Achieve Similar Results as Combination Therapy for IBD? (2019)
- Proactive Therapeutic Drug Monitoring -Different Time Points
- Improving Outcomes with Proactive Therapeutic Drug Monitoring + Swiss COVID-19 Data
- Real-World Experience with Proactive Therapeutic Drug Monitoring in Inflammatory Bowel Disease (2021) [This blog post summarizes reference #24 in the current study]

