BESands, et al. Inflammatory Bowel Diseases, 2024. 30: 2024 2245–2258. Open Access! Two-Year Efficacy and Safety of Mirikizumab Following 104 Weeks of Continuous Treatment for Ulcerative Colitis: Results From the LUCENT-3 Open-Label Extension Study
In this LUCENT-3 study, the authors examined response at 2 years among patients who had response to treatment at 1 year; patients received 200 mg mirikizumab every 4 weeks. The authors stratified patients by induction response and by previous biologic exposure.
Key findings (from Figure 4):
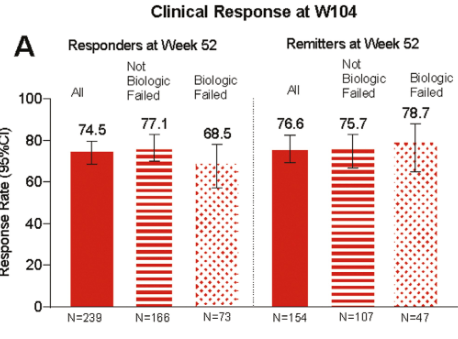
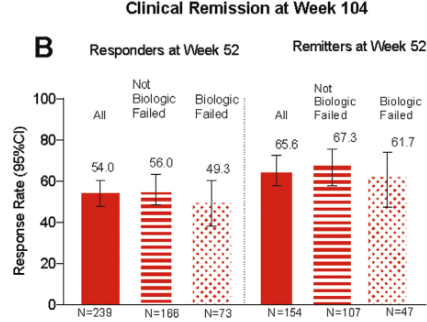
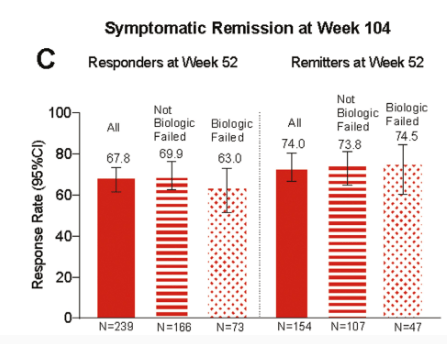
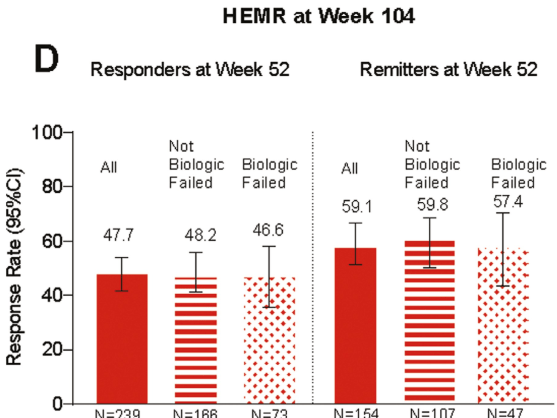
- No new safety signals were identified, and the discontinuation rate due to adverse events was 2.8%
My take: It is good to see extended data for mirkizumab. Head-to-head trials, though, are needed to better determine which therapies are most effective.
Related blog posts:
