DT Rubin et al. Lancet 2024; 405: 33-49. Guselkumab in patients with moderately to severely active ulcerative colitis (QUASAR): phase 3 double-blind, randomised, placebo-controlled induction and maintenance studies
Background: “Guselkumab is a dual-acting, human IgG1, interleukin-23p19 subunit inhibitor that potently neutralises interleukin-23 and can bind to CD64.”
Methods: “Two phase 3, randomised, double-blind, placebo-controlled studies (QUASAR phase 3 induction and maintenance) included randomised and treated adults with moderately to severely active ulcerative colitis (induction baseline modified Mayo score from 5 to 9) with inadequate response or intolerance to conventional or advanced ulcerative colitis therapy.”
“The induction study primary population included 701 patients (guselkumab 200 mg given intravenously 60% [421 patients]; placebo 40% [280 patients]). The maintenance study primary population included 568 guselkumab induction responders randomly assigned to receive guselkumab 200 mg given subcutaneously every 4 weeks (190 [33%] patients) or 100 mg every 8 weeks (188 [33%] patients) or placebo (guselkumab withdrawal 190 [33%] patients).”
Key findings:
- INDUCTION DATA AT 12 WEEKS:
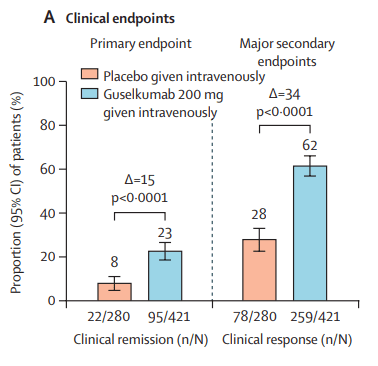
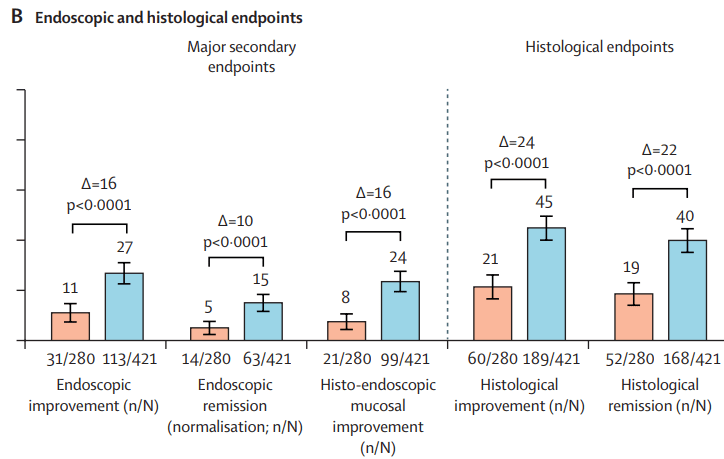
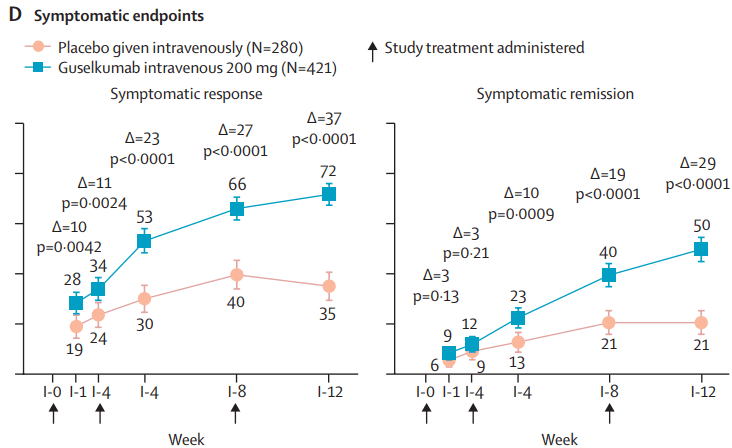
MAINTENANCE DATA AT 44 WEEKS
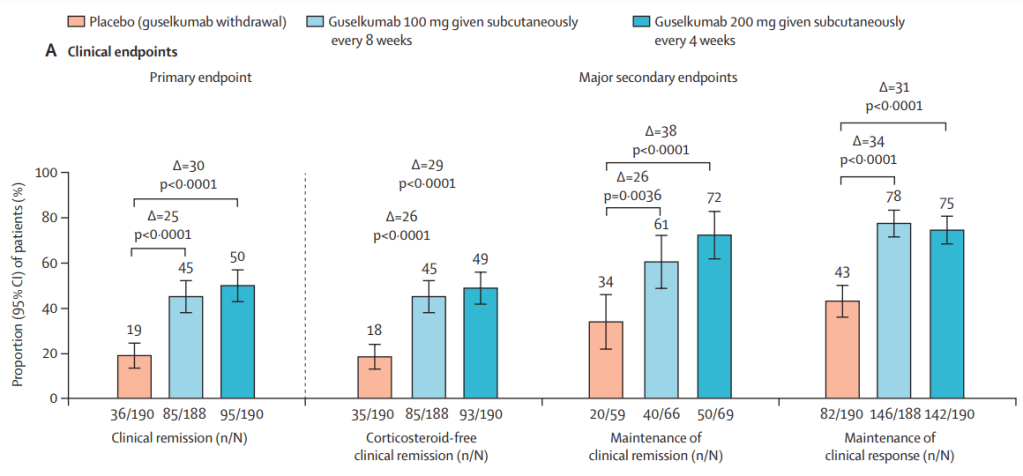
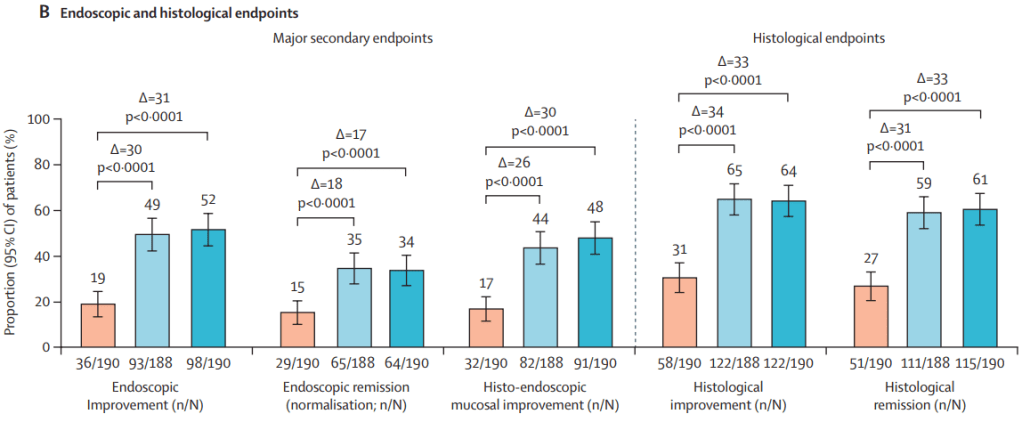
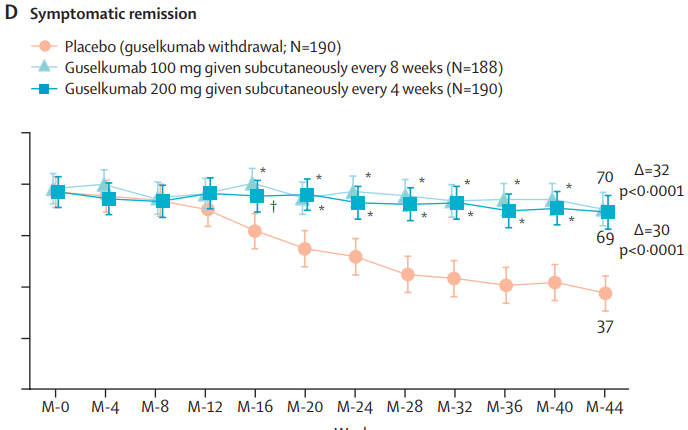
Results and Discussion points:
- “Symptomatic improvement was observed as early as induction week 1 (first assessed timepoint)”
- “Greater reductions in C-reactive protein and faecal calprotectin concentrations with guselkumab induction compared with placebo were observed as early as induction week 4 (first assessed timepoint)”
- “Guselkumab efficacy was shown in both biologic naive and JAK inhibitor-naive patients, and in patients with a history of inadequate response or intolerance to biologics or JAK inhibitors”
- “Overall, 34% (129 of 378) of patients in the guselkumab groups achieved endoscopic
remission at maintenance week 44. Among the 180 patients in the guselkumab groups in clinical remission at maintenance week 44, 124 (69%) of 180 were in endoscopic remission” - “Symptomatic remission and deep symptomatic remission achieved with guselkumab induction was generally maintained to maintenance week 44 with guselkumab relative to placebo”
- “The incidences of anti-guselkumab antibodies and NAbs were low in both the induction and maintenance studies…titres were low and did not affect serum concentration, efficacy, or safety”
- “Head-to-head comparison data with other IL-23 antagonists are currently not available”
- “Safety results were consistent with the known and favourable safety profile of guselkumab in its approved indications. Rates of adverse events, serious adverse events, and adverse events leading to treatment discontinuation generally did not occur more frequently in patients treated with guselkumab versus placebo-treated patients”
- Limitation: The primary analysis population for the maintenance study included only guselkumab induction responders following 12 weeks of intravenous treatment
My take: Overall, this is a pivotal study showing that guselkumab is an effective agent for moderately to severely active ulcerative colitis in those with and without prior treatments. More head-to-head studies are needed to determine the optimal positioning of therapies for UC. Currently, AGA guidelines (AGA Living Guideline for Moderate-to-Severe Ulcerative Colitis –The Good and The Bad) suggest that guselkumab should be considered in the top tier of medications used in patients naive to biologics/advanced therapies and in the second tier for those with prior biologic treatments.
Related blog posts:
- New IBD Medication: Guselkumab for UC (QUASAR study) (Phase 2b study)
- Comparative Evidence and Positioning Advance Therapies for Inflammatory Bowel Disease)
- Dr. Joel Rosh: Positioning Therapies for Pediatric Ulcerative Colitis (2024)
- IBD Updates: Insurance Barriers Hindering Care, Guselkumab vs Ustekinumab, IBD Pain Management Guidelines
- Guselkumab: Expanding the GALAXI of Treatments for Crohn’s Disease
- Risankizumab for Ulcerative Colitis
