LA Aronne et al. NEJM 2025; DOI: 10.1056/NEJMoa2416394. Tirzepatide as Compared with Semaglutide for the Treatment of Obesity
Methods: In this phase 3b, open-label, controlled “SURMOUNT-5” trial, adult participants (n=751) with obesity but without type 2 diabetes were randomly assigned in a 1:1 ratio to receive the maximum tolerated dose of tirzepatide (10 mg or 15 mg) or the maximum tolerated dose of semaglutide (1.7 mg or 2.4 mg) subcutaneously once weekly for 72 weeks
Key findings:
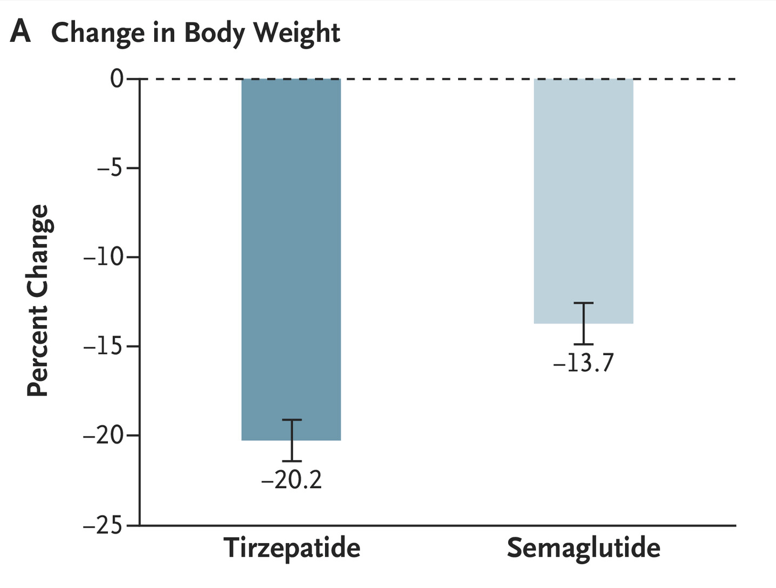
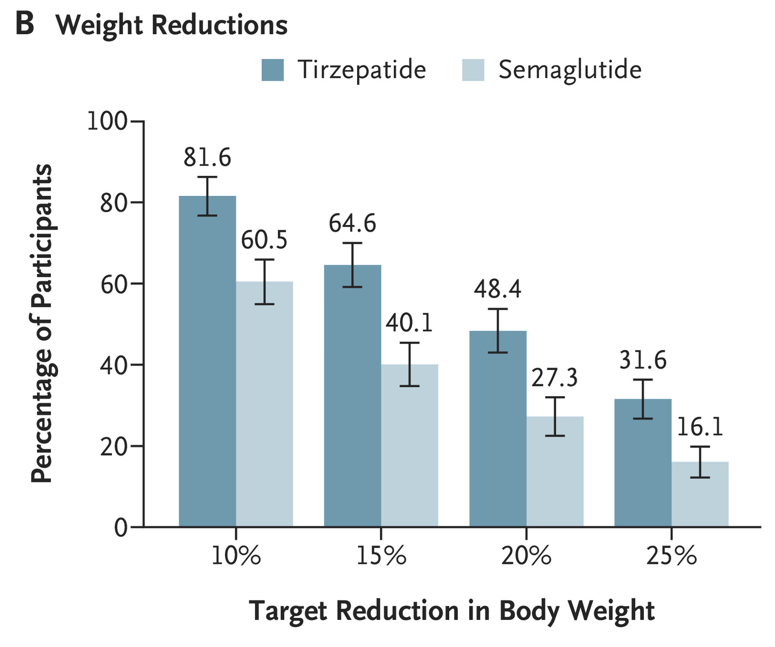
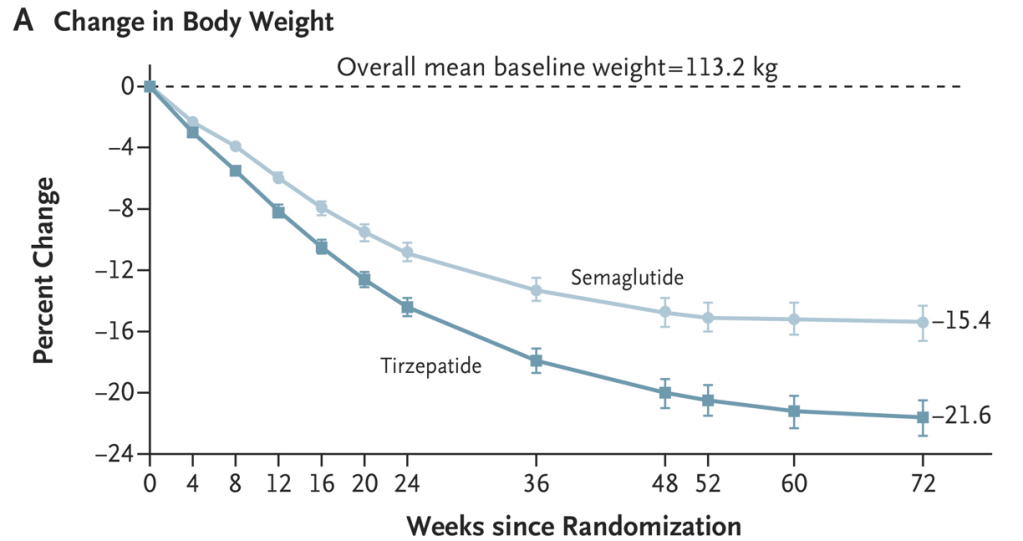
Discussion Points:
“With both treatments in our trial, as weight reduction increased, greater improvements occurred in cardiometabolic risk factors, including blood pressure, glycemia, and lipid levels, which is consistent with the findings in previous reports.17 The mean differences between tirzepatide and semaglutide in the cardiometabolic risk factors may be clinically relevant considering that reductions in systolic blood pressure of 2 to 5 mm Hg have been shown to reduce the risk of cardiovascular events.”
” As typically observed with incretin-based therapies, gastrointestinal adverse events were predominantly mild to moderate in severity, occurred mostly during dose escalation, and led to treatment discontinuation more often with semaglutide than with tirzepatide.”
My take (borrowed from the authors): “Treatment with tirzepatide, a dual GIP and GLP-1 receptor agonist, was superior to treatment with semaglutide, a selective GLP-1 receptor agonist, with respect to reduction in body weight and waist circumference.”
Related blog posts:
- Lifetime Health Effects and Cost-Effectiveness of Tirzepatide and Semaglutide in US Adults
- Tirzepatide: Breakthrough in Obesity and Diabetes Management (SURMOUNT-1 Study at 3 years)
- Tirzepatide for Metabolic Dysfunction–Associated Steatohepatitis (MASH) & Uptick in GLP1 Use
- Key Insights on MASLD from Dr. Marialena Mouzaki
- Semaglutide Keeps Weight Off at Four Year Mark
- Semaglutide in Adolescent Obesity
- Pharmacological Management of Pediatric Steatotic Liver Disease
- More Data Indicating GLP-1 Efficacy for MASH
