G Pellegatta et al. Clin Gastroenterol Hepatol 2025; 23: 1058-1060. Open Access! Switch From Off-Label Swallowed Topical Corticosteroids to Budesonide Orodispersible Tablets in Eosinophilic Esophagitis Patients
Methods: This was a single center, prospective, observational study with adult patients previously diagnosed with EoE. Thirty EoE patients, receiving off-label swallowed topical corticosteroids (STCs), were consecutively enrolled. “This is the first study to evaluate the clinical, histological, and endoscopic efficacy of the switch from STCs to BOT [Budesonide Orodispersible Tablet]”
Key findings:
- The median Dysphagia Symptoms Score decreased from 5 (range 0–9) under STCs therapy to 0 (range 0–6) under BOT therapy (P < .0001)
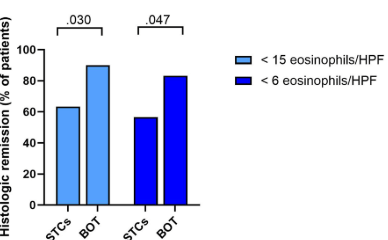
- After switching to BOT, there was a significant increase in the number of patients in histological remission (STCs: n = 19 of 30 [63.3%] vs BOT: n = 27 of 30 [90%]; P = .030) and histological deep remission (STCs: n = 17 of 30 [56.6%] vs BOT: n = 25 of 30 [83.3%]; P = .047)
- Another important improvement following the switch was the improved patient satisfaction with the therapy in terms of a faster and easier modality of assumption…favors a better compliance to BOT
- There was a “slight increase in oral Candida infection after BOT”
The authors did not include any cost information regarding the switch. In U.S., BOT is not an available treatment option. However, Eohilia, which is a budesonide suspension with a 12-week FDA approval period, costs ~$2100 per month (for 600 mL =30 day supply), whereas budesonide ampules at same dosage cost ~$300 per month (60 1 mg ampules).
My take: BOT therapy, which was targeted for esophageal delivery, was associated with better response rates. However, the cost of targeted FDA approved budesonide therapy in U.S, is exorbitant.
Related blog posts:
- Orodispersible Budesonide Tablets for Eosinophilic Esophagitis
- Delivery Vehicle and Outcomes for Budesonide-Treated Eosinophilic Esophagitis
- Budesonide FDA-Approved for Eosinophilic Esophagitis
- Budesonide for Maintaining EoE Remission
- Head-to-Head: Budesonide vs Fluticasone for Eosinophilic Esophagitis
- Budesonide Looks Better for Eosinophilic Esophagitis
- Maintenance Topical Steroid Dosing for Eosinophilic Esophagitis
