R Panaccione et al. The Lancet. Published online July 17, 2025 https://doi.org/10.1016/S0140-6736(25)00681-6. Efficacy and safety of intravenous induction and subcutaneous maintenance therapy with guselkumab for patients with Crohn’s disease (GALAXI-2 and GALAXI-3): 48-week results from two phase 3, randomised, placebo and active comparator-controlled, double-blind, triple-dummy trials
Methods: “GALAXI-2 and GALAXI-3 were identically designed, phase 3, randomised, double-blind, triple-dummy, treat-through trials with active and placebo comparators…1048 participants were randomly assigned, treated, and followed up until week 48, of whom 1021 participants were included in the primary analysis population: 508 (49·8%) in GALAXI-2 and 513 (50·2%) in GALAXI-3.” The studies enrolled adult patients with moderately to severely active Crohn’s disease.
Key findings:
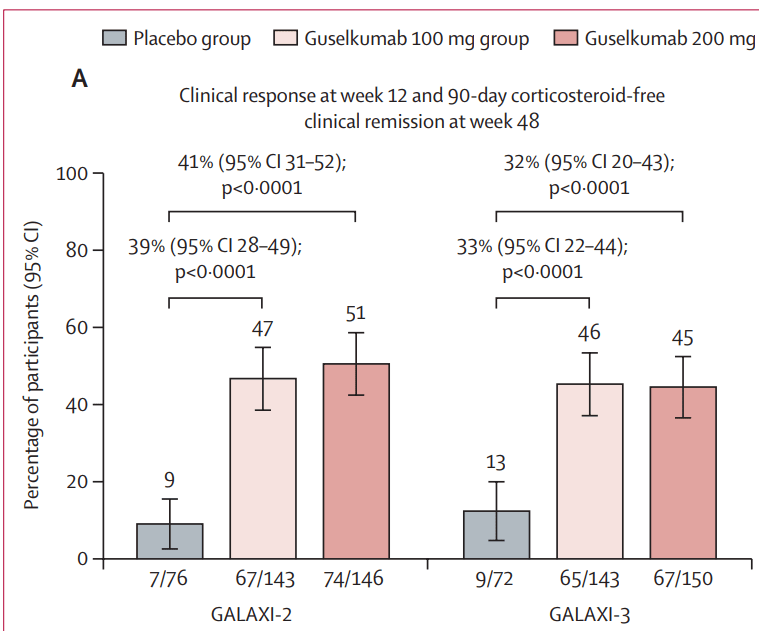
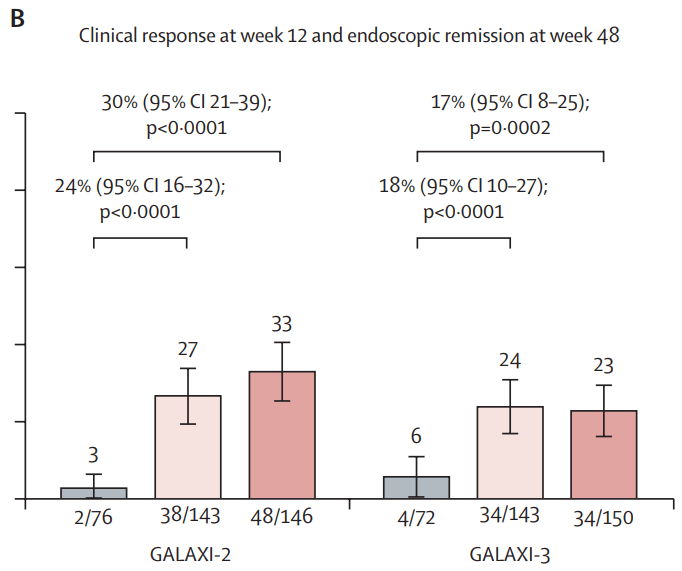
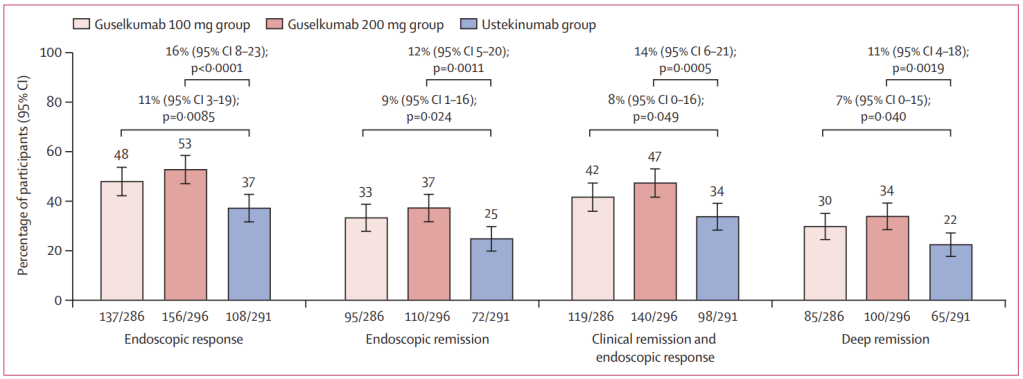
Discussion points:
- “Guselkumab treatment in participants with moderately to severely active Crohn’s disease was also evaluated in the GRAVITI study, which had a fully subcutaneous induction and maintenance treatment regimen. Clinical and endoscopic outcomes reported with subcutaneous guselkumab induction in the GRAVITI study were similar to those in the phase 3 GALAXI studies following intravenous guselkumab induction.”
- “The incidence of adverse events with guselkumab during induction was low and similar to placebo.”
My take (borrowed from authors): In GALAXI-2 and GALAXI-3, both guselkumab dose regimens (each including intravenous induction and subcutaneous maintenance) were superior to placebo for short-term (week 12) and long-term (week 48) endpoints and both guselkumab dose regimens were also superior to ustekinumab
Related blog posts:
