D’Alise, A.M., Willis, J., Duzagac, F. et al. Nat Med (2026). https://doi.org/10.1038/s41591-025-04182-9. Nous-209 neoantigen vaccine for cancer prevention in Lynch syndrome carriers: a phase 1b/2 trial. (Open Access!)
Background: “Lynch syndrome (LS) is a prevalent hereditary cancer syndrome affecting ~1 in 300 individuals, with an overall lifetime cancer risk as high as 80%. LS is caused by germline mutations in the DNA mismatch repair genes, leading to microsatellite instability (MSI) and accumulation of shared mutations. When these occur in coding regions, they generate frameshift peptides (FSPs). Nous-209 is a neoantigen-directed immunotherapy” against these FSPS. These are “the results from cohort 1 of a phase 1b/2 single-arm trial of Nous-209 for cancer interception in LS carriers (n = 45).”
Key findings:
- Neoantigen-specific immune responses were observed after vaccination in 100% of evaluable participants (n = 37), with induction of potent T cell immunity
- The immune response was durable and detectable at 1 year in 85% of participants
- Both CD8+ and CD4+ T cells were induced, recognizing multiple FSPs
- Peptide–human leukocyte antigen predictions allowed the identification of >100 immunogenic FSPs with demonstration of cytotoxic activity in vitro
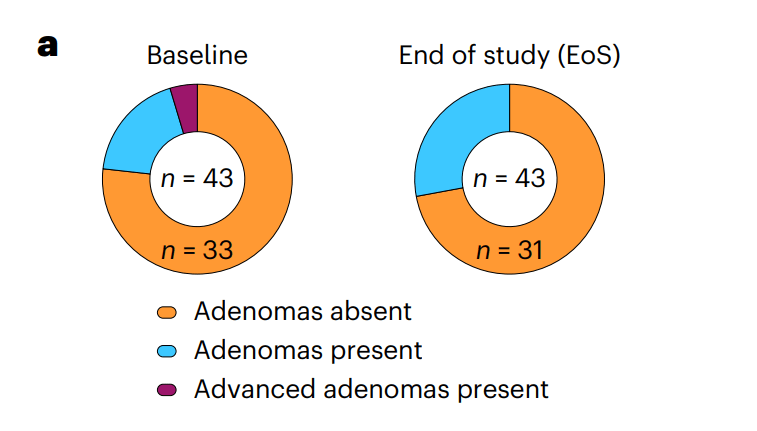
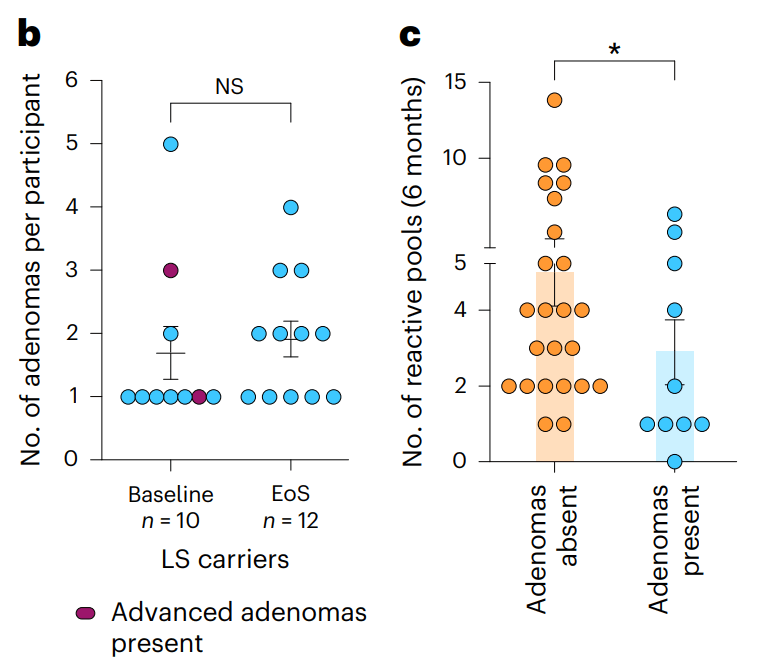
My take (borrowed in part from authors): “Overall, this clinical trial provides important proof-of-concept data of the safety and the robustness of induced immunogenicity of
Nous-209 in LS carriers…and supporting its clinical development as a valuable intervention for cancer immune interception.” Vaccines have a long history in reducing cancer (for Hepatitis B, Cervical Cancer (due to HPV), Anal Cancer, Leukemia (by boosting immunity) and Others). Until recently, this has been by preventing viral infections that increase the risk of cancer. This is a new approach.
Related article: Blood test for colorectal cancer: A Mannucci et al. Gastroenterol 2026; 170: 330-343. An Exosome-Based Liquid Biopsy for the Detection of Early-Onset Colorectal Cancer: The ENCODER Multicenter Study Methods: A panel of 6 cell-free and exosome-based circulating biomarkers were identified through small RNA sequencing from a biomarker discovery cohort (blood test). Key finding: “This study developed and independently tested a blood-based test with 97.3% sensitivity for screening-relevant CRC stages I–III and 61.5% for the noninvasive detection of high-grade dysplasia.”
Related blog posts:
- From Treatment to Cures for Autoimmune Diseases
- Vaccination Can Lower the Risk of a Childhood Cancer
- Good News Story: The Remarkable Hepatitis B Vaccine Story
- Image Only: Eliminating Cervical Cancer with HPV Vaccine
- Another Reason For HPV Vaccine –Prevention of Anal Cancer
- Genetic Risk Assessment and Testing for Gastrointestinal Cancers and Polyposis (2025)
- Dr. Steve Erdman: Perplexing Polyposis Patients: a Case-Based Discussion
