J Goldstein, NY Times, 12/1/25: The Transgender Cancer Patient and What She Heard on Tape
This article focuses on the experience of a transgender patient who recorded unfavorable remarks while under anesthesia.
An excerpt:
On the recording, the health care workers express a variety of opinions about transgender identity more generally….And in the middle of the conversation, one person suggests updating Ms. Capasso’s medical file. “Yeah, it needs to say ‘male,’” the person says.Ms. Capasso said it appeared that hospital staff had in fact changed her electronic medical records, all while she was unconscious…
Ms. Capasso insists that she was not trying to catch the medical staff speaking disrespectfully about her. She said she was motivated by curiosity and a desire to know exactly what the surgeons discovered. It may not be such an unusual impulse.
Dr. Alexander Langerman, a surgeon at Vanderbilt University Medical Center, led a medical conference in 2021 on surgical recordings.
There is often “a really strong desire by patients to know what happened to them in the operating room,” Dr. Langerman said. “And, I think, a valid right to know what happened.”
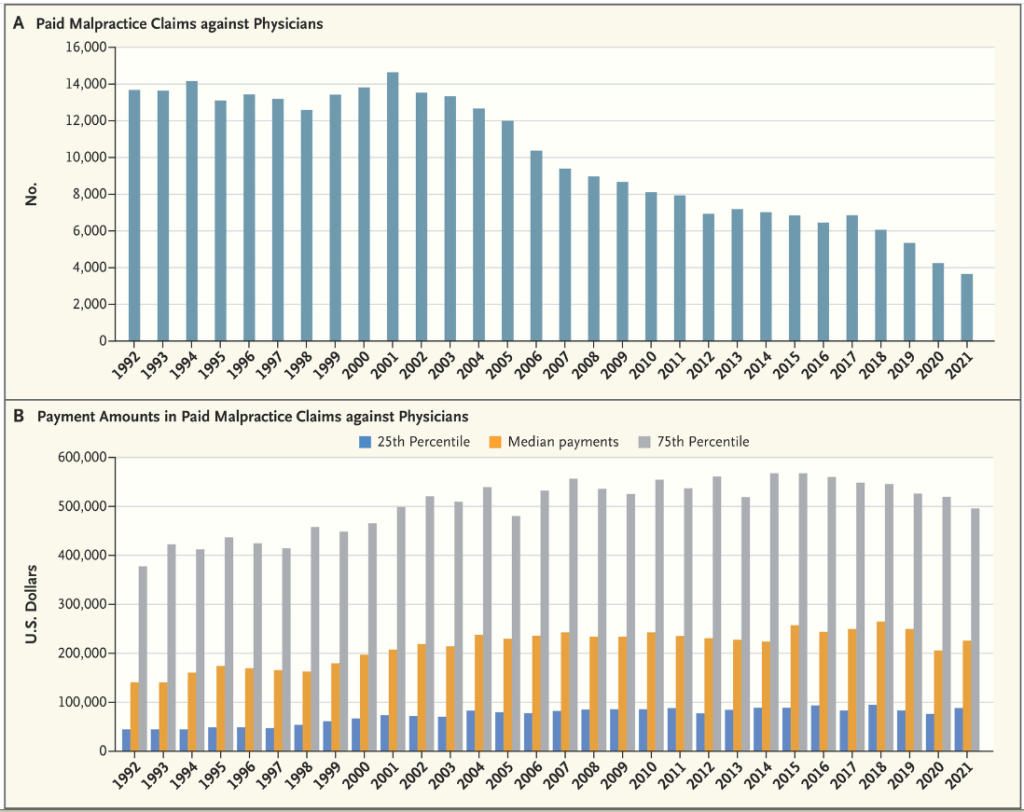
Surgery was once a relatively public event — operating rooms were called “theaters” for a reason. But infection control and malpractice litigation pulled the operating room out of public view.
“Operating rooms and surgery have become one of the most secretive environments in modern society,” said Dr. Teodor Grantcharov, a Stanford University professor who started a company that uses operating room recordings to improve patient safety and hospital efficiency.
My take: While a patient is under anesthesia, it is best to treat them in the same manner as if they were awake.
“The true test of a man’s character is what he does when no one is watching.”
― John Wooden