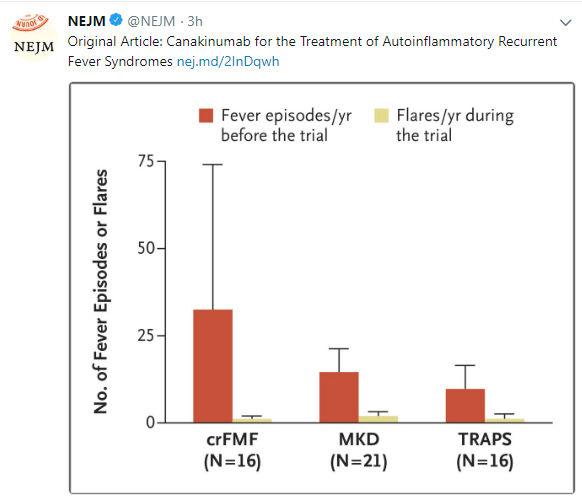
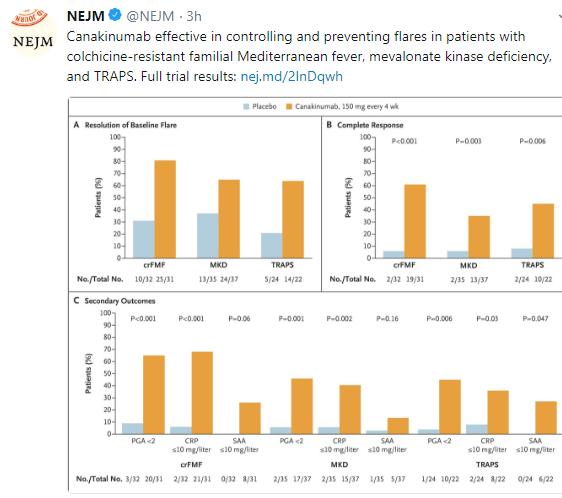
A conference report on periodic fever, aphthous stomatitis, pharyngitis, adenitis syndrome (PFAPA): L Harel et al. J Pediatr 2018; 193: 265-74
This report reviews PFAPA along with other fever syndromes.
Table II reviews several published criteria. Most of these include abrupt onset of fever, duration of symptoms <5 days, presence of constitutional symptoms, exclusion of cyclic neutropenia, presence of aphthous stomatitis, pharyngitis, cervical adenitis, presence of asymptomatic intervals, normal growth.
- The authors note that ~25% of patients are >5 years of age.
- They note that it is important to exclude exudative tonsillitis.
- They suggest NOT testing for familial Mediterranean fever (FMF) in the absence of clinical suspicion. The pain symptoms with FMF are much more intense and consistent with a peritonitis.
- They recommend checking acute phase reactants between attacks to assure normalization
- Corticosteroids (single dose) have been shown to shorter course. “The recommended full dose is 2 mg/kg prednisone or 0.3 mg/kg betamethasone.”
- “It is our practice to conclude the following: 1. Fever recurring the next day [after steroids]–not a PFAPA episode, 2. fever recurring withing 2-4 days –the corticosteroid dose is too low, and 2. attack recurs >1 week –new episode.”
- Any of the following should exclude PFAPA: “neutropenia, cough, coryza, severe abdominal pain, significant diarrhea, rash, arthritis, or neurologic abnormalities; elevated acute phase reactants between attacks”
Differential diagnosis and characteristics are reviewed in Figure 5, with emphasis on mevalonate kinase deficiency, FMF, cryopyrin-associated periodic syndromes (CAPS), and tumor necrosis factor receptor-associated periodic syndrome (TRAPS).
Related blog posts:
Disclaimer: These blog posts are for educational purposes only. Specific dosing of medications/diets (along with potential adverse effects) should be confirmed by prescribing physician/nutritionist. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.

View from Bright Angel Trail