EV Loftus et al. N Engl J Med 2023; 388:1966-1980. Upadacitinib Induction and Maintenance Therapy for Crohn’s Disease
This study is the basis for the FDA’s approval of updacitnib (Rinvoq) for Crohn’s disease in adults: New FDA Rinvoq (upadacitinib) Indication: Oral Treatment For Crohn’s
This publication describes the results of two multicenter, double-blind, randomized, placebo-controlled induction trials (n=1021 adults,U-EXCEL, U-ECEED) and one maintenance trial (n=502, U-ENDURE) with Upadacitinib (Rinvoq). The induction trials involved an early mandatory glucocorticoid taper.
Key findings:
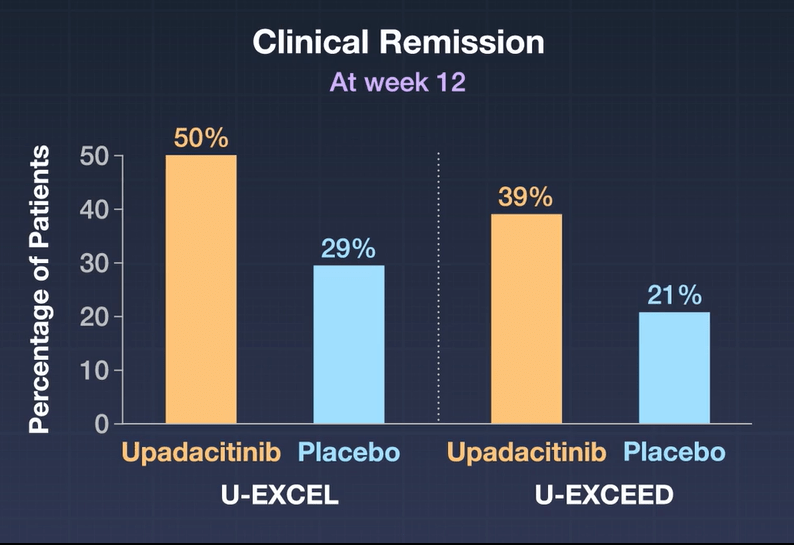
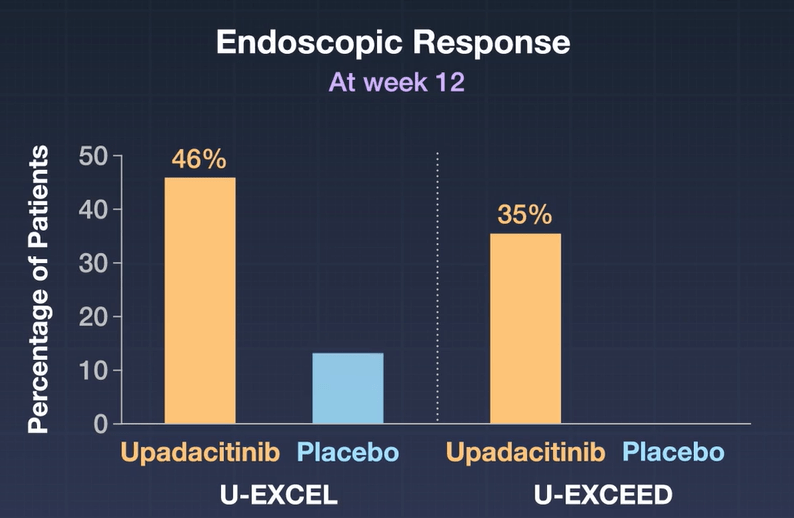
- A significantly higher percentage of patients who received 45-mg upadacitinib than those who received placebo had clinical remission (in U-EXCEL, 49.5% vs. 29.1%; in U-EXCEED, 38.9% vs. 21.1%) and an endoscopic response (in U-EXCEL, 45.5% vs. 13.1%; in U-EXCEED, 34.6% vs. 3.5%) (P<0.001 for all comparisons).
- There was a rapid onset of action with a difference in clinical response compared to placebo at 2 weeks
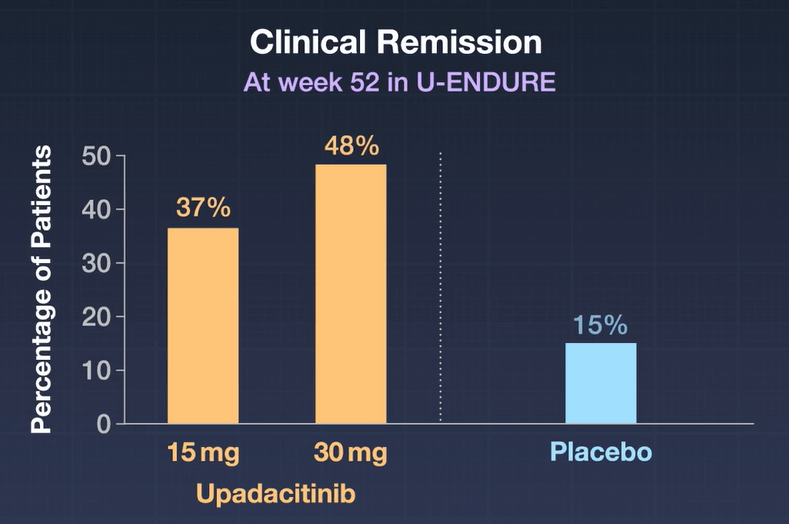
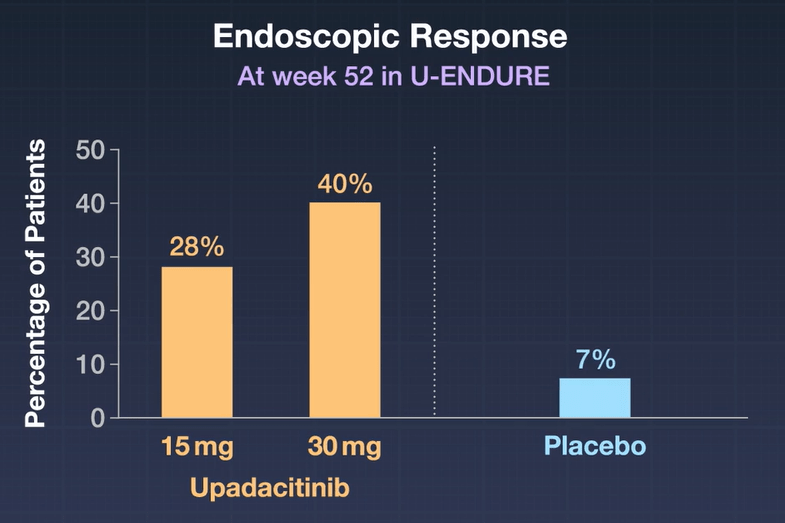
- Maintenance Trial of clinical responders: At week 52 in U-ENDURE, a higher percentage of patients had clinical remission with 15-mg upadacitinib (37.3%) or 30-mg upadacitinib (47.6%) than with placebo (15.1%), and a higher percentage had an endoscopic response with 15-mg upadacitinib (27.6%) or 30-mg upadacitinib (40.1%) than with placebo (7.3%) (P<0.001 for all comparisons).
- Adverse effects included gastrointestinal perforations (6 in study medication, 1 in placebo), neutropenia in up to 2.6%, and increased Herpes Zoster infections in patients receiving study medication (1.5% to 3%).
A good commentary of this study is in the same issue: M Abreu. N Engl J Med 2023; 388:2005-2009. It is noted that upadacitinib showed a good response even though a different JAK inhibitor, tofacitinib, had disappointing results for patients with Crohn’s disease. Other points:
- “It is hard to compare findings across studies because of differences in the characteristics of patients and end points. That being said, the incidences of clinical remission observed by Loftus et al. were greater than those observed in most studies of biologic drugs to treat Crohn’s disease. Moreover, upadacitinib was more likely than placebo to resolve extraintestinal manifestations.”
- “They did not find evidence of cardiovascular or thromboembolic complications, which were previously observed in patients with rheumatoid arthritis treated with tofacitinib and which led to a black-box warning.10 However, the treatment of greater numbers of patients for a longer duration will be required to determine whether upadacitinib is asssociated with a risk of such complications.”
- “Among the most common upadacitinib-specific adverse events were anemia [6.9%] and acne [6.3%]. The increase in anemia may be due to off-target effects of upadacitinib on erythropoietin signaling through JAK2.”
My take: This is great news for patients with Crohn’s disease. In addition to having a new option for refractory disease, this option does not require IV administration. When will pediatric data be available?
