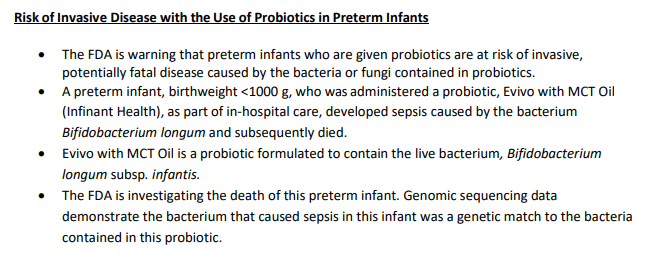
9/29/23 FDA: Risk of Invasive Disease in Preterm Infants Given Probiotics Formulated to
Contain Live Bacteria or Yeast
“The FDA cautions that microorganisms contained in probiotics have been reported in the medical literature as causing bacteremia or fungemia, sometimes with a severe clinical course, in very preterm or very low birthweight (VLBW) infants.”
“Moreover, the American Academy of Pediatrics states ‘Given the lack of FDA-regulated pharmaceutical grade products in the United States, conflicting data on safety and efficacy, and potential for harm in a highly vulnerable population, current evidence does not support the routine, universal administration of probiotics to preterm infants, particularly those with a birth weight of <1000 g.’”
“The FDA is also reminding healthcare providers that FDA has not approved any probiotic product for use as a drug or biological product in infants.”
My take: Despite promising studies (over the last 20 years) indicating that probiotics reduce the risk of necrotizing enterocolitis and death, this appears to be the end of the line for the use of probiotics for preemies in the U.S. To change course, there would need to be a pharmaceutical-grade probiotic and proof that it was a reliable high-quality agent meeting FDA standards along with subsequent studies showing this probiotic was effective.
Reference: Poindexter B. Use of probiotics in preterm infants. Pediatrics 2021;147(6):June 2021:e2021051485. https://publications.aap.org/pediatrics/article/147/6/e2021051485/180282/Use-of-Probiotics-in-PretermInfants
Related blog posts:
- What About the Risk of Not Intervening to Prevent Necrotizing Enterocolitis?
- Probiotics in Preemies: Lifesaving Therapy Lots of studies have indicated that probiotics may be beneficial in premature newborns; the problem is that there are currently no FDA-approved probiotics for preterm infants. The use of probiotics as a non-regulated FDA product leads to the potential risk of contamination due to inconsistent quality control as well as variability in the strains and concentrations. The risks are not inconsequential as there has been a report of 29-week infant who died from mucormycosis due to probiotic contamination with mold.
- Can Necrotizing Enterocolitis Be Prevented with Antibiotics?
- Probiotics -Another Positive Study for Prevention of Necrotizing Enterocolitis
- Probiotics for NEC -More Work is Needed (part 2)
- Probiotics For NEC -More Work is Needed (part 1)
