AJ Sanyal et al. NEJM 2024; DOI: 10.1056/NEJMoa2401755. A Phase 2 Randomized Trial of Survodutide in MASH and Fibrosis
Background/Methods: “Dual agonism of glucagon receptor and glucagon-like peptide-1 (GLP-1) receptor may be more effective than GLP-1 receptor agonism alone for treating metabolic dysfunction–associated steatohepatitis (MASH). The efficacy and safety of survodutide (a dual agonist of glucagon receptor and GLP-1 receptor) in persons with MASH and liver fibrosis” was studied in “48-week, phase 2 trial, we randomly assigned adults with biopsy-confirmed MASH.” (n=293)
“Dual agonism of glucagon receptor and GLP-1 receptor may be more effective than GLP-1 receptor monoagonism for treating MASH, because the extrahepatic benefits of GLP-1 receptor agonism (glucose control, reduced appetite, and weight loss) are combined with direct hepatic effects (increased energy expenditure, lipolysis, and mobilization of hepatic fat) associated with glucagon receptor agonism.”
Key findings:
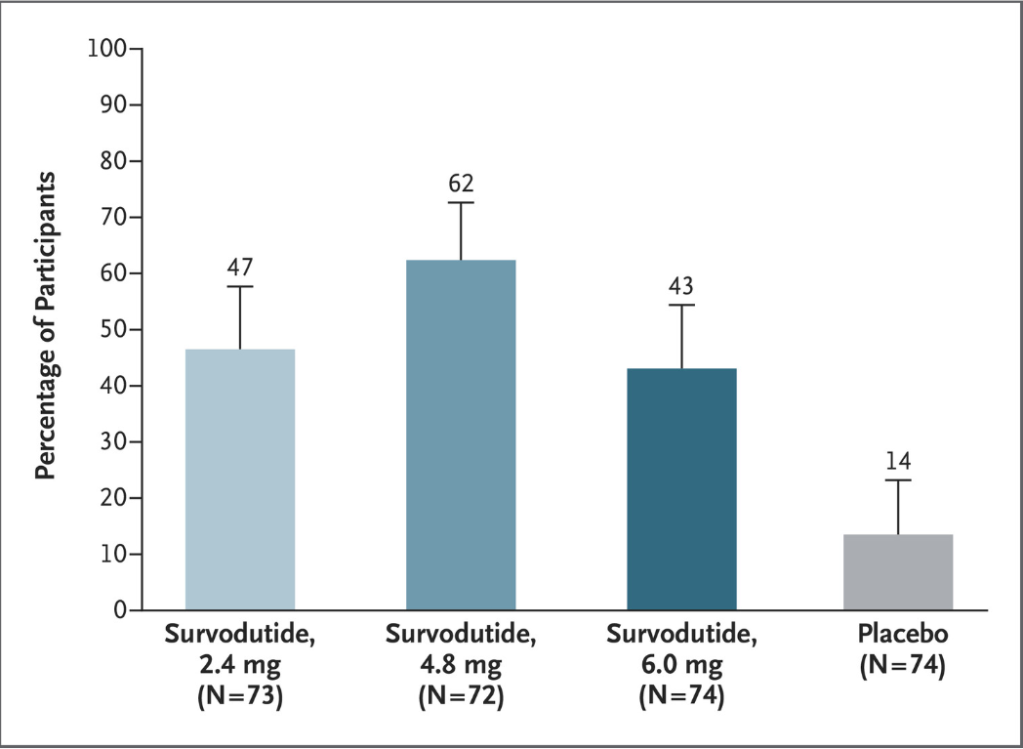
- Improvement in MASH with no worsening of fibrosis occurred in 47% of the participants in the survodutide 2.4-mg group, 62% of those in the 4.8-mg group, and 43% of those in the 6.0-mg group, as compared with 14% of those in the placebo group
- A decrease in liver fat, assessed by MRI-PDFF, content by at least 30% occurred in 63% of the participants in the survodutide 2.4-mg group, 67% of those in the 4.8-mg group, 57% of those in the 6.0-mg group, and 14% of those in the placebo group; improvement in fibrosis by at least one stage occurred in 34%, 36%, 34%, and 22%, respectively.
- Adverse effects were more frequent with survodutide than with placebo included nausea (66% vs. 23%), diarrhea (49% vs. 23%), and vomiting (41% vs. 4%); serious adverse events occurred in 8% with survodutide and 7% with placebo.
The discussion notes that “in a phase 2 trial, treatment with the GLP-1 receptor monoagonist semaglutide resulted in a significantly higher percentage of patients with MASH resolution than placebo but not in a significantly higher percentage of patients with improvement in fibrosis stage.26” Thus, the improvement in fibrosis, which was seen in this study with survodutide,will need to be examined in future studies.
My take: Earlier this year, the selective thyroid hormone receptor beta agonist resmetirom gained conditional approval from the Food and Drug Administration as the first pharmacotherapy for MASH with moderate-to-advanced liver fibrosis.5 It looks like there will be a number of pharmacologic agents available in the coming years. Cost and availability will be ongoing concerns. In addition, determining when/how these agents will be used in the pediatric population will not be clear for quite a long time.
Related blog posts:
- Resmetirom for MASH
- Resmetirom (Rezdiffra) -FDA Approved for MASH with Moderate to Advanced Fibrosis
- You No Longer Have Fatty Liver Disease-You Have Steatotic Liver Disease!
- Good Review on Newest Medications for Obesity
- Online Aspen Webinar (Part 6) -NAFLD and NASH
- Fatty Liver Feast (of Articles): NAFLD 2020
- ACG Review (Zobair Younassi, MD): NAFLD and NASH
