A Zinger et al. Clin Gastroenterol Hepatol 2024; 22: 1336-1338. Risankizumab Effectiveness and Safety in Crohn’s Disease: Real-world Data From a Large Tertiary Center
In a group of 80 patients with Crohn’s disease with evidence of active disease, the authors examined the effectiveness of risankizumab with prospectively-collected data. Patients received 600 mg intravenously at 0, 4, and 8 weeks. Only 6 patients (8%) were unexposed to prior advanced therapy; 44 patients (55%) had prior ustekinumab (UST) therapy.
Key findings:
- Clinical remission was 78% in patients without prior ustekinumab therapy and 64% in those with prior ustekinumab therapy
- Steroid-free clinical remission was 75% and 52%, respectively in patients without and with prior ustekinumab therapy. Overall, 63% of patients achieved a steroid-free clinical remission
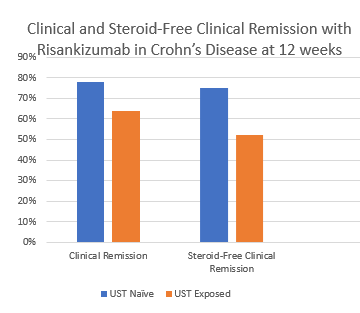
My take: This study shows that risankizumab, a selective IL23 inhibitor, has good effectiveness, even in patients previously treated with a IL12/23 inhibitor. It highlights our need to better understand the reasons why a more selective agent is able to work after patients failed to respond to UST treatment.
Unrelated article: KA Chien et al. JPGN 2024; 79: 10-17. (Kudos to the authors including my partner Dr. Ben Gold). This article detailed the median work RVUs target for practices and composition of healthcare team to provider ratios: Nursing 0.80, MA 0.29, dietician 0.29, social worker 0.14, and psychologist 0.13. The article reviews salary structure/incentives and wellness initiatives as well.
Related blog posts:

Pingback: Crohn’s Disease: Risankizumab Real-World Data - reviewer4you.com