JHW Zhang et al. NEJM 391: 2098-2109. Xalnesiran with or without an Immunomodulator in Chronic Hepatitis B
Background: Xalnesiran, a small interfering RNA molecule that targets a conserved region of the hepatitis B virus (HBV) genome and silences multiple HBV transcripts.
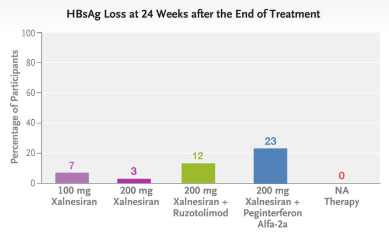
Methods: This was a phase 2, multicenter, randomized, controlled, adaptive, open-label platform trial that included the evaluation of 48 weeks of treatment with xalnesiran at a dose of 100 mg (group 1), xalnesiran at a dose of 200 mg (group 2), xalnesiran at a dose of 200 mg plus 150 mg of ruzotolimod (group 3), xalnesiran at a dose of 200 mg plus 180 μg of pegylated interferon alfa-2a (group 4), or a nucleoside or nucleotide analogue (NA) alone (group 5) in participants with chronic HBV infection who had virologic suppression with NA therapy. The primary efficacy end point was hepatitis B surface antigen (HBsAg) loss (HBsAg level, <0.05 IU per milliliter) at 24 weeks after the end of treatment.
Key findings:


In the associated editorial by Janssen et al. (NEJM 2024;391:2163-2168), the authors note that “nucleoside or nucleotide analogues currently form the backbone of therapy for most patients with chronic HBV infection who have access to treatment…However, treatment must be continued on a long-term basis…Although treatment with nucleoside or nucleotide analogues is associated with a decrease in the severity of liver fibrosis and reduction of liver-related complications, the risk of hepatocellular carcinoma persists at the population level because functional cure is not achieved in most patients.”
“Further data are needed on the durability of the effect achieved with new agents that directly interfere with HBsAg production. The results reported by Hou et al. indicate a risk of relapse, undermining the choice of functional cure as an end point for these agents, at least when assessed relatively early after the withdrawal of therapy…Hou et al. observed that functional cure was achieved only in patients with a baseline HBsAg level of less than 1000 IU per milliliter. Although a substantial proportion of patients have similarly low HBsAg levels, patients with higher HBsAg levels have the greatest risk of adverse liver-related outcomes and thus have the most to gain from new therapies.”
My take: While this study represents important progress, it is not likely to change current treatment strategies in the near-term. Even better than treating HBV is preventing HBV. The best strategy for reducing HBV mortality and morbidity still relies of wide-scale use of the highly effective HBV vaccine.
Related blog posts:
- New Age for Hepatitis B Therapies
- Is It Time to Revise Hepatitis B Treatment Guidelines?
- Current Practice in Hepatitis B and Long-term Prospects
- What’s New in the Treatment of Hepatitis B
- Good News Story: The Remarkable Hepatitis B Vaccine Story
- Withdrawal of Chronic Hepatitis B Therapy
- Lessons Learned from Children In the Hepatitis B Virus Research Network
