R Panaccione et al. Clinical Gastroenterology and Hepatology 2025; In press. Open Access! Extended Risankizumab Treatment in Patients With Crohn’s Disease Who Did Not Achieve Clinical Response to Induction Treatment
Addendum -updated reference: R Panaccione et al. Clinical Gastroenterology and Hepatology 2025; 23: 2012-2022. Open Access! Extended Risankizumab Treatment in Patients With Crohn’s Disease Who Did Not Achieve Clinical Response to Induction Treatment
Methods: Per the study design, patients who did not achieve SF/APS clinical response following induction could receive 12 weeks of extended treatment with RZB, either via administration of the higher (1200 mg) IV RZB dose evaluated in ADVANCE and MOTIVATE or by initiation of SC RZB at doses (180 mg and 360 mg) used in FORTIFY maintenance therapy.
Key findings:
- Over 60% of initial nonresponders achieved clinical response with extended RZB treatment. These patients also demonstrated improved clinical and endoscopic outcomes during the extended treatment period, which were sustained or continued to improve during maintenance.



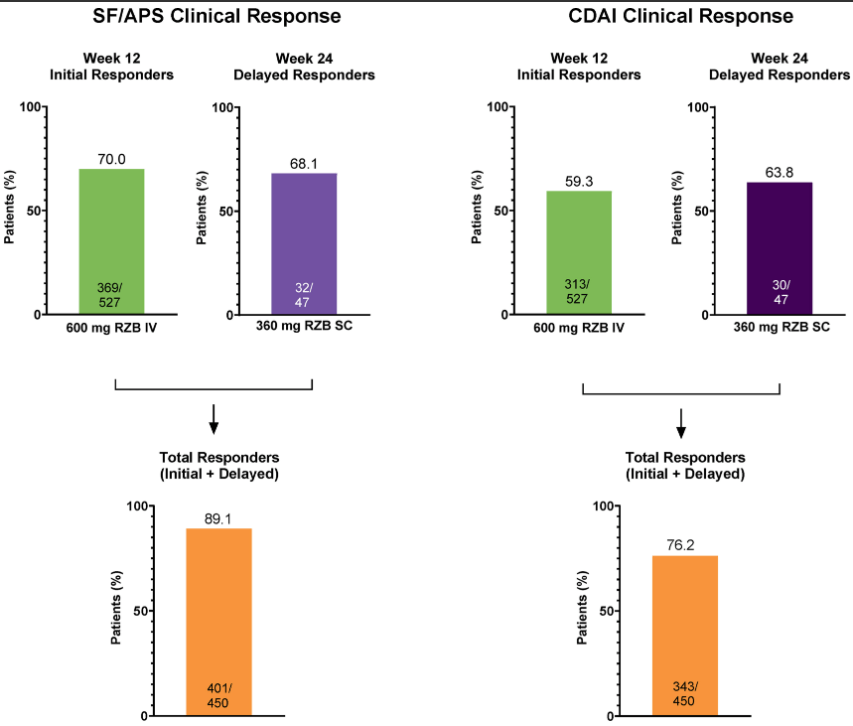
My take: While there is a very good response with initial risankizumab therapy in Crohn’s disease, it looks like judgment on response needs to wait until 24 weeks as there are many who do not respond at 12 weeks who will subsequently respond to treatment.
Related blog posts:
- Dr. Maria Oliva-Hemker: Positioning Therapies for Pediatric Crohn’s Disease
- Comparative Evidence and Positioning Advance Therapies for Inflammatory Bowel Disease
- Risankizumab Outperforms Ustekinumab
- Crohn’s Disease: Risankizumab Real-World Data
- Impressive Results for Risankizumab in Refractory Crohn’s Disease
- Risankizumab Receives FDA Approval for Crohn’s Disease
