EB Mitchell et al. JPGN 2025;80:653–663. Ustekinumab is safe and effective in pediatric patients with Crohn’s disease
This was a retrospective longitudinal cohort study of 101 children with CD treated with ustekinumab from two large centers between 2015 and 2020. The median follow-up time on ustekinumab was 16.6 months.
Key findings:
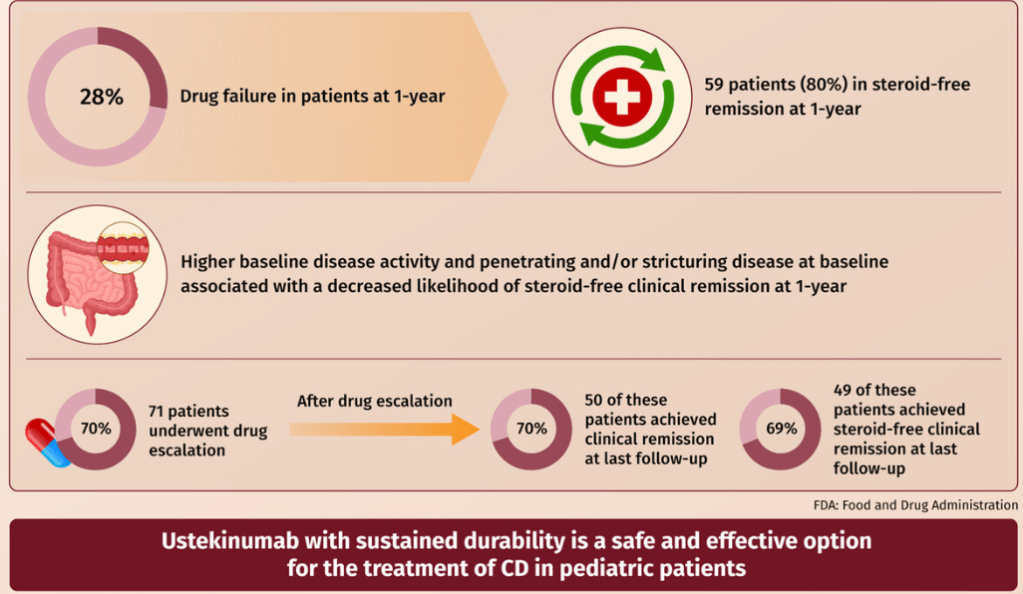
- Fifty-nine patients were in steroid-free clinical remission at 1 year.
- Higher baseline disease activity (odds ratio [OR]: 0.91 (p = 0.01) and stricturing/penetrating disease phenotype (OR: 0.14 p = 0.02) were associated with decreased likelihood of steroid-free clinical remission at 1-year
- Ustekinumab drug escalation occurred in 70% of patients, and after escalation, 50 (70%) achieved clinical remission, and 49 (69%) achieved steroid-free remission at the last follow-up
- Adverse events were rare and did not require therapy discontinuation
My take: More pediatric data showing efficacy for ustekinumab is important. My sense, though, is that newer IL-23 specific agents are going to eclipse ustekinumab in pediatrics as they are doing in adults.
Related blog posts:
- Dr. Maria Oliva-Hemker: Positioning Therapies for Pediatric Crohn’s Disease
- Ustekinumab Data at 4 Years -UNIFI Extension Study
- Pediatric Data for Ustekinumab Therapy in Crohn’s Disease
- Risankizumab Outperforms Ustekinumab
- Comparative Efficacy: Infliximab vs. Ustekinumab
- FDA Approves Ustekinumab Biosimilar Pyzchiva
- IBD Updates: Insurance Barriers Hindering Care, Guselkumab vs Ustekinumab, IBD Pain Management Guidelines