R Panaccione et al. Clin Gastroenterol Hepatol 2025; (In press) Open Access! Upadacitinib Maintenance Therapy in Crohn’s Disease: Final Results From the Randomized Phase 3 U-ENDURE Study
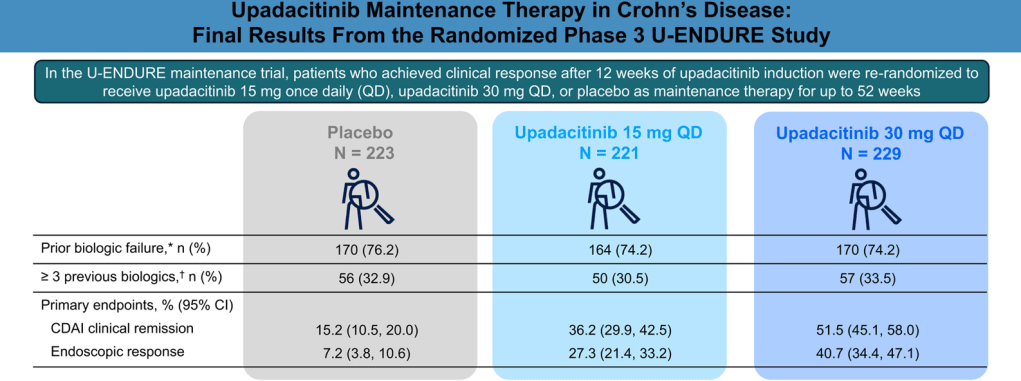
Methods: Clinical responders to 12 weeks of upadacitinib 45 mg once daily (QD) induction were randomized (1:1:1) to receive upadacitinib 15 mg QD (n = 221), upadacitinib 30 mg QD (n = 229), or placebo (n = 223) as maintenance therapy for 52 weeks
**This study presents data from the entire cohort (n=673); a previous report from ENDURE-3 analyzed data on 502 patients (though findings were nearly identical). EV Loftus et al. N Engl J Med 2023; 388:1966-1980 (Related post: Landmark Study: Oral Biologic for Crohn’s –Upadacitinib)
Key findings:
- At week 52, more upadacitinib-treated vs placebo patients achieved CDAI clinical remission (upadacitinib 15 mg, 36.2% and upadacitinib 30 mg, 51.5% vs placebo, 15.2%)
- The rates of endoscopic response were 27.3% for upadacitinib 15 mg and 40.7% for upadacitinib 30 mg vs 7.2% for placebo
- Herpes zoster infections occurred more frequently in the upadacitinib groups compared with placebo; all were nonserious, and most involved a single dermatome
- In U-ENDURE, no dose-dependent risk for MACE, VTE, or malignancy (excluding NMSC) was observed during the 52-week maintenance period


My take: Upadacitinib is a effective in a good number of patients with with moderately to severely active Crohn’s disease who have been refractory to other advanced therapies.
Related blog posts:
- Upadacitinib’s Effectiveness for Perianal Fistulizing Crohn’s Disease
- How Quickly Does Upadacitinib Work for Crohn’s Disease Symptoms?
- IBD Briefs: Upadacitinib in Children, Predicting Crohn’s Disease, and Autoimmune Diseases Associated with IBD
- Landmark Study: Oral Biologic for Crohn’s –Upadacitinib
