S Langreen et al. JPGN 2025;81:626–633. Rectal budesonide: A potential game changer after Kasai hepatoportoenterostomy
Background: After the START trial in 2014, it seemed that enthusiasm for post-operative steroids for biliary atresia had waned. The START study did not find that steroids improved outcomes after Kasai hepatoportoenterostomy (HPE). Subsequently, though, there have been observational reports of using steroids in a customized fashion to improve outcomes. Langreen et al add to this literature by examining their use of rectal budesonide (2 mg) for 3 months in a retrospective cohort (n=142) with a historical control (n=137). Jaundice-free native liver survival (jfNLS) was assessed at 6 months, 2 years, 5 years, and 10 years post-Kasai.
Key findings:
- Improvements were noted in jfNLS at 6 months (53% vs. 39%) , 2 years (45% vs. 22%), 5 years (40% vs. 23%) and 10 years (32% vs. 13%)
- These benefits were exclusive to patients with nonsyndromic BA
- No serious adverse effects were identified with budesonide
Rationale for rectal budesonide: The authors note that “a single dose of budesonide foam contains about 2 mg of budesonide, equivalent to 25 mg of prednisolone or 20 mg of methylprednisolone…In our series, no serious steroid associated adverse effects were recorded, possibly due to the first pass after rectal administration.”
Limitations: “The retrospective nature of our data analysis allows for variability in the follow‐up protocols, potential biases (historical control group, change of surgeons) and confounding factors cannot be entirely ruled out.”
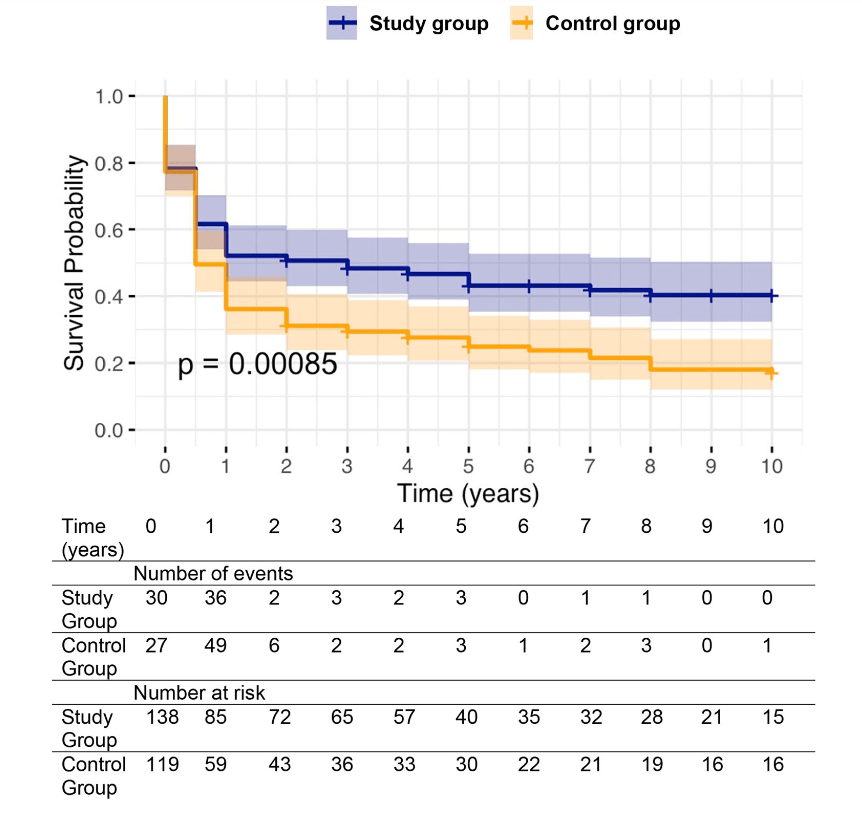
over a 10‐year follow‐up.. Study group—blue. Control group—orange.
My take: The START study with 140 participants was well-designed and did not find a benefit with systemic steroids. However small differences in outcomes can be difficult to identify. Rectal budesonide may improve outcomes. A randomized, double-blind, placebo-controlled trial would be more definitive.
Related blog posts:
