From Consumer Reports, April 18, 2025: We Tested 41 Baby Formulas for Lead and Arsenic
This Consumer Reports article is likely to generate a lot of attention. Thanks to Dr. Seth Marcus for sharing this reference.
An excerpt:
While some formulas had concerning levels [of arsenic and lead], there are safer choices. After seeing our results, the FDA is pledging further action…
Consumer Reports tests in the past have found elevated levels of inorganic arsenic in fruit juice, baby food, and bottled water…Our tests found the highest inorganic arsenic level in Abbott Nutrition’s EleCare Hypoallergenic, at 19.7 parts per billion (ppb), and the second highest in Similac Alimentum at 15.1 ppb, also made by Abbott.
As we had expected, CR’s tests found lead in almost all the formulas. Lead levels ranged from 1.2 ppb to 4.2 ppb, which is below the FDA’s Closer to Zero goal, but CR’s experts believe those levels are too high...
Together the formula made by these three companies—Abbott, Mead Johnson, and Perrigo—makes up 79 percent of the U.S. market…They also said trace levels of heavy metals in the food supply are not an issue that is unique to infant formula…
Perrigo, which makes Dr. Brown’s formula and many popular store brands we tested, including Kirkland, Parent’s Choice, Member’s Mark, and Up&Up, also told us that it routinely screens its formulas for heavy metals. “These compounds and PFAS are also found in breast milk,” a spokesperson wrote. “Their levels in infant formula are insignificant and well below regulations in the United States and around the world.”
Contaminants from the environment pose a problem for our entire food supply, CR experts say. But the problem is much more urgent for formula, given how vulnerable babies who depend on it are.
The FDA has long been limited by a lack of both resources and authority to carry out all the oversight it’s tasked with. ..
Keep these test results in perspective. Environmental pollutants are pervasive in our food supply, and all the contaminants in our tests—arsenic, lead, BPA, acrylamide, and PFAS—have also been previously detected in breast milk, food, and water…
Never ever try to make your own baby formula or offer alternative foods. It’s unsafe from a nutrition standpoint…
Use clean water to mix into your powdered formula. The EPA sets limits on contaminants in tap water for most of the country, but not every part of it. If you drink water from a well, for instance, that water is not regulated by the EPA. So it’s a good idea to get well water tested for heavy metals and PFAS before using it…
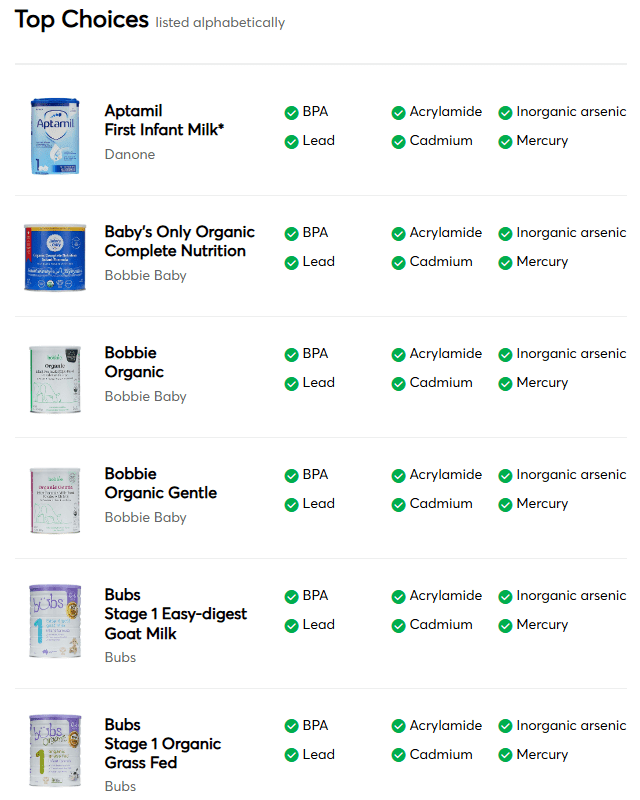
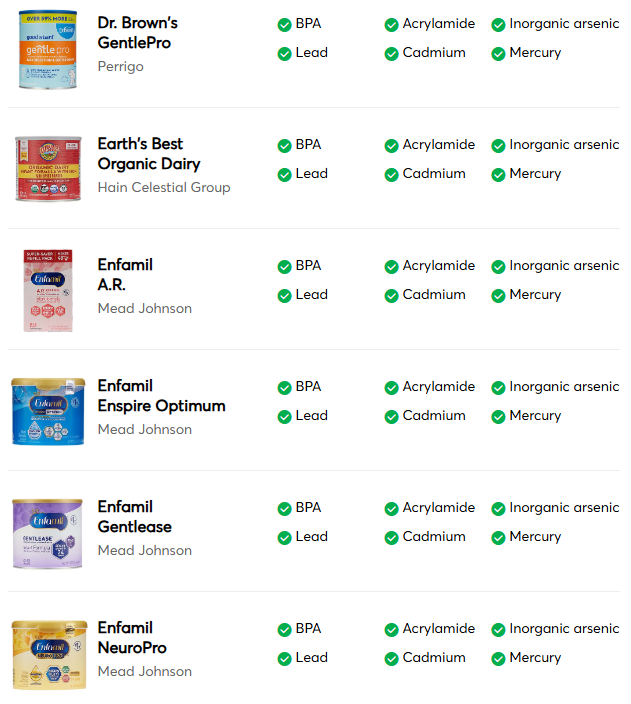
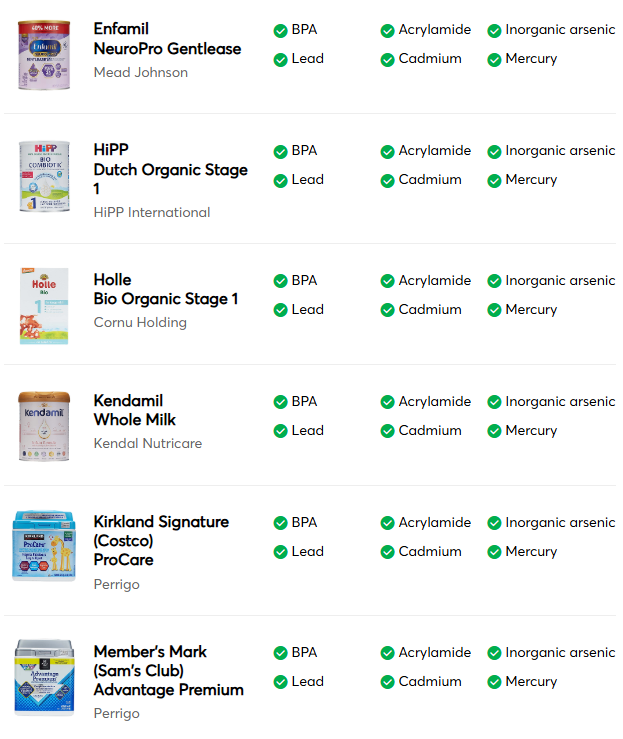

“Good Choices”
- A2 Platinum -A2 Milk Company
- ByHeart Whole Nutrition -ByHeart
- Happy Baby Organics -Danone
- Kendamil Organic -Kendal Nutricare
- Neocate Hypoallergenic -Danone
- Parent’s Choice Infant -Perrigo
- Similac 360 Total Care -Abbott Nutrition
- Similac 360 Total Care Sensitive -Abbott Nutrition
- Similac Sensitive -Abbott Nutrition
- Similac Soy Isomil -Abbott Nutrition
“Worse Choices”
- Dr. Brown’s SoothePro -Perrigo
- Elecare Hypoallergenic -Abbott Nutrition
- Enfamil Nutramagen -Mead Johnson
- Enfamil ProSobee Simply Plant-Based -Mead Johnson
- Kabrita Goat Milk-Based -Ausnutria
- PurAmino Hypoallergenic -Mead Johnson
- Similac Alimentum -Abbott Nutrition
- Similac NeoSure -Abbott Nutrition
- Similac Total Comfort -Abbott Nutrition
- Up&Up (Target) Soy -Perigo
My take: Formula companies need to continue to work on minimizing all of the contaminants. Yet, if all families selected only CR’s “top choices,” there would not be enough formula for infants who are not breastfed. In addition, this problem is even more of an issue in children needing specialized hypoallergenic formulas.
Related blog posts:
- Ensuring Safe Infant Formula Use -More Complicated Than You Think
- “Pediatric Formula Basics”
- Overdiagnosis of Cow’s Milk Protein Allergy in Infants and Formula Industry Influence
- WIC Formula Selection in Infants and Children
- Where Will We Be Without Formulas for Premature Babies?
- Proliferation of Formula Lawsuits In Necrotizing Enterocolitis


