- RK Sterling et al. Hepatology 2025; 81: 321-357. Open Access! AASLD Practice Guideline on blood-based noninvasive liver disease assessment of hepatic fibrosis and steatosis
- K Patel et al. Hepatology 2025; 81: 358-379. Open Access! Review: Accuracy of blood-based biomarkers for staging liver fibrosis in chronic liver disease: A systematic review supporting the AASLD Practice Guideline
This guideline reviews and recommends blood-based tests as a tool to help determine the likelihood/severity of liver fibrosis in the presence of chronic liver disease. Most of the guideline focuses on adult liver disease. For pediatrics, the guideline makes the following recommendation:
In the pediatric patients with chronic liver disease, AASLD suggests the use of simple, cost-effective, and readily available blood-based NILDA [Non-invasive Liver Disease Assessment], such as APRI or FIB-4, for the detection of advanced fibrosis (F3-4) (ungraded statement).
Technical Remarks:
- Some blood-based NILDA in children have good accuracy in detecting advanced fibrosis but have difficulty discriminating earlier stages of fibrosis.
- FIB-4 does not perform as well in children as it does in adults, particularly very young children, due to the inclusion of age in the index.
- Rapid growth in children and attendant fluctuations in alkaline phosphatase can confound interpretation of blood or collagen-based NILDA tests in pediatric liver disease.
- There are insufficient biopsy validated data to recommend biomarkers for evaluating fibrosis in pediatric NASH and α1AT at this time.
- In the pediatric population with CLD, there is growing but insufficient evidence to recommend blood-based NILDA as endpoints to monitor changes in fibrosis over time.
Despite the guidance recommendation, reading the text makes one leery about relying on these tests:
- For example with biliary atresia: “The utility of APRI to assess or predict liver fibrosis in BA is mixed in the current literature.”
- “In conclusion, blood-based NILDA tests in children vary widely in their accuracy, even in detecting F3-4 fibrosis, and have difficulty discriminating earlier stages of fibrosis. These tests also have different disease-specific thresholds that correlate with histopathologic fibrosis and differ from adults. APRI and FIB-4 have been the most studied NILDA tests in children, but there is still insufficient evidence to recommend blood biomarkers as endpoints to monitor changes in fibrosis over time. Any blood-based NILDA that includes age (Table 5) should be used cautiously in children.“
My take: This practice guideline, while recommending use of blood-based tests for fibrosis even in the pediatric age group, makes a fairly compelling argument that they are unreliable in children. Elastrography is likely to be more useful, though also imperfect, in the pediatric population.
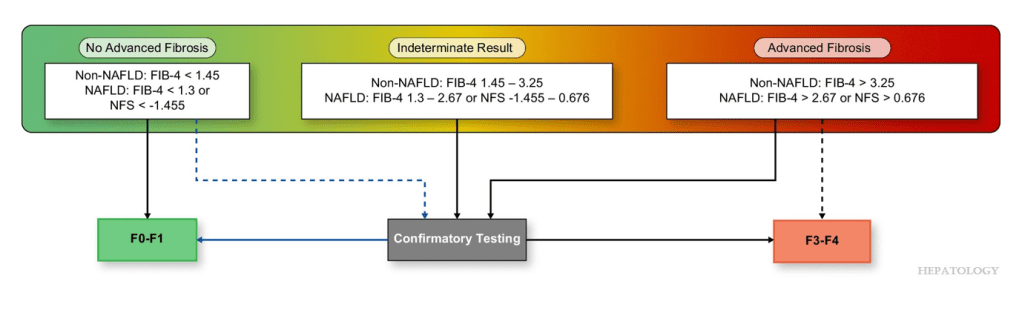
Algorithm Recommended for Adults:
Related blog posts:



