MV Vestergaard et al. . JAMA. Published online October 15, 2024. doi:10.1001/jama.2024.20429. HLA-DRB1*01:03 and Severe Ulcerative
Colitis
Background: This study aimed to identify biomarkers by conducting a Danish nationwide genome-wide association study (GWAS) on severe vs less severe ulcerative colitis.
Methods: Severe ulcerative colitis: Patients with severe ulcerative colitis were defined as having at least 1 major ulcerative colitis–related operation, at least 2 ulcerative colitis–related hospitalizations exceeding 2 days, and/or use of at least 5000 mg of systemic corticosteroids within 3 years of diagnosis
The authors utilized two source populations
- The Center for Molecular Prediction of Inflammatory Bowel Disease (PREDICT) neonatal blood spot cohort (NBS) includes individuals born in Denmark and diagnosed with ulcerative colitis from 1981 to 2022
- The North Denmark Biobank study is a population-based cohort of patients from Northern Denmark with inflammatory bowel disease from 1978 to 2020 (NorDIBD)
The combined cohort included 4491 patients (4153 from NBS and 338 from NorDIBD) with a mean (SD) age at diagnosis of 23.3 (8.4) years; 53% of patients were female and 27% had severe disease.
Key findings:
- The association with HLA-DRB1*01:03 (Figure 1) had an OR of 6.38 for major operation, OR of 5.24 for at least 2 hospitalizations, and OR of 2.30 for use of at least 5000 mg
of systemic corticosteroids in carriers vs noncarriers - Carriage of HLA-DRB1*01:03 allele was 2.8% in these cohorts
- Limiation: Danish cohort -may not be applicable to other populations
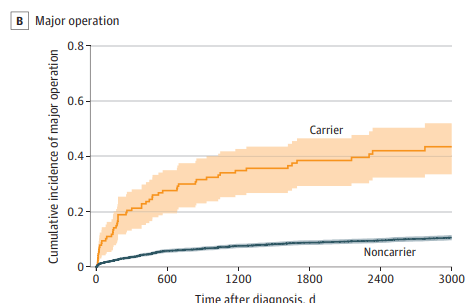
My take: HLA-DRB1*01:03 is a low-frequency allele, carriers have a significantly higher risk of severe ulcerative colitis.
Related blog posts:
- Does Accelerated Dosing of Infliximab Work for Acute Severe Ulcerative Colitis?
- Comparative Evidence and Positioning Advance Therapies for Inflammatory Bowel Disease)
- Dr. Joel Rosh: Positioning Therapies for Pediatric Ulcerative Colitis (2024)
- AGA Living Guideline for Moderate-to-Severe Ulcerative Colitis –The Good and The Bad
- IBD Updates: SMART IBD App, SC Vedolizumab Durability, Risk Factors in Acute Severe Ulcerative Colitis
- Bridge Therapy for Ustekinumab with Acute Severe Ulcerative Colitis
- ARCH Study: Higher Doses of Infliximab in Acute Severe Ulcerative Colitis
- @MondayNightIBD and Acute Severe Ulcerative Colitis Algorithm
- An Overlooked Finding in a Recent Acute Severe Ulcerative Colitis Study
- Vedolizumab vs Adalimumab: Histology Outcomes from Varsity Trial, Vedolizumab More Effective Than Adalimumab for Ulcerative Colitis
- CCFA 2023 (Atlanta) -Part 1
- CCFA 2023 (Atlanta) Part 4
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.
