AN Ananthakrishnan, J Adler et al. Gastroenterol 2023; 165: 1367-1399. Open Access! AGA Clinical Practice Guideline on the Role of Biomarkers for the Management of Crohn’s Disease
Key points:
- Recommendation #2: In patients in symptomatic remission with recent endoscopic evaluation (w/in 3 yrs), a fecal calprotectin <150 μg/g and normal CRP rules out active inflammation, avoiding endoscopic evaluation for assessment of disease activity. However, elevated biomarkers in this setting merit confirmation with endoscopy before treatment adjustment.
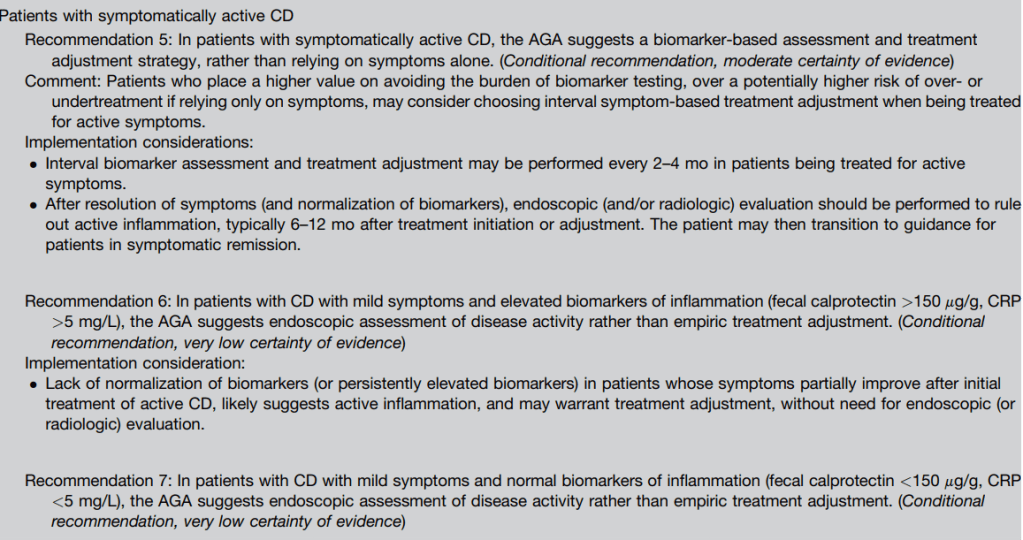
- Recommendations #6, #7: In patients with CD with mild symptoms, neither normal nor elevated biomarkers alone are sufficiently accurate to determine endoscopic activity.
- Recommendations #8, #9: In patients with CD with moderate to severe symptoms, elevated fecal calprotectin or serum CRP suggests endoscopic activity, precluding routine endoscopic assessment for disease activity. In those with moderate to severe symptoms but normal biomarkers, endoscopic assessment is recommended rather than empiric adjustment in treatment.
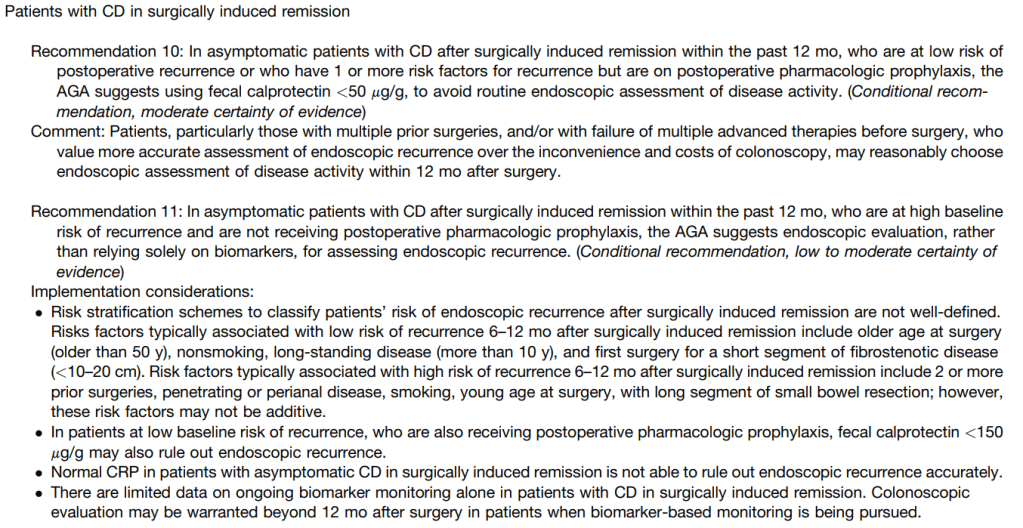
- Recommendation #10: In patients with CD in surgically induced remission in low-risk patients on pharmacologic prophylaxis, a normal fecal calprotectin (<50 mcg/gm) reliably rules out endoscopic recurrence.
More Recommendations:
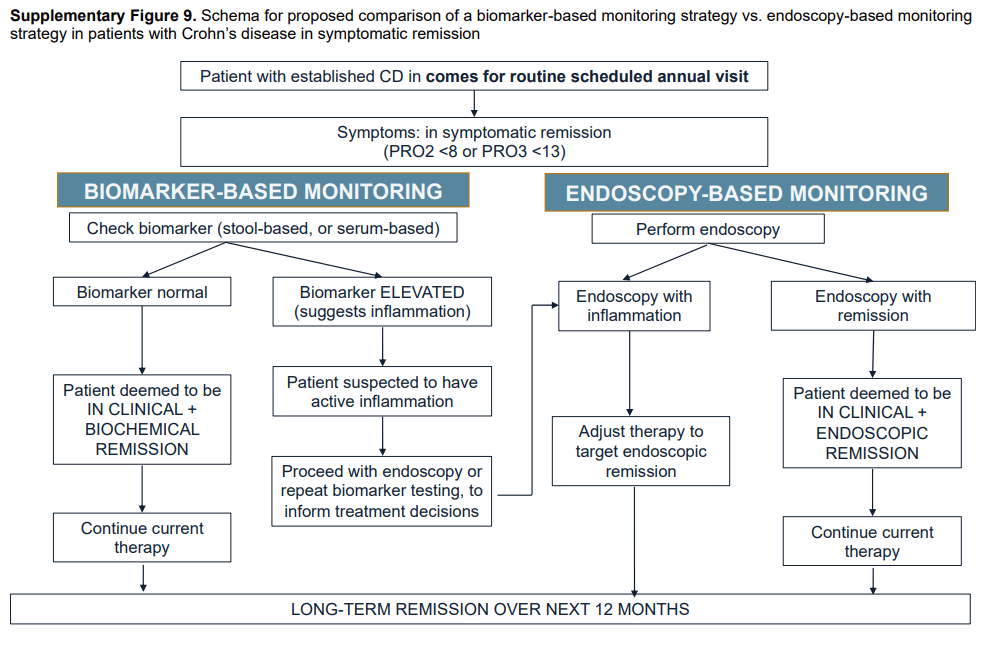
#1 In patients with CD in symptomatic remission, the AGA suggests a monitoring strategy that combines biomarkers and symptoms, rather than relying on symptoms alone.
#3 In patients with CD in symptomatic remission without recent confirmation of endoscopic remission, the AGA suggests endoscopic evaluation to rule out active inflammation, rather than relying solely on fecal calprotectin or CRP.
#5 In patients with symptomatically active CD, the AGA suggests a biomarker-based assessment and treatment adjustment strategy, rather than relying on symptoms alone.
My take: This practical guidance will help target endoscopy in patients with Crohn’s disease. In those who are feeling well with normal biomarkers, frequent endoscopic evaluation is a low-value procedure. Similarly, in those with very elevated biomarkers and who are very symptomatic (with normal infectious studies), endoscopic evaluation is often unnecessary. The AGA expert recommendations should help persuade insurance companies to include biomarkers in their coverage.
Summary of all recommendations -see below from Figure 9 and Table 3.


Related blog posts:
- Guideline: Biomarker Use for Ulcerative Colitis (2023)
- What is the Calprotectin Threshold for Disease Progression in Crohn’s Disease?
- Correlating Calprotectin with Disease Severity in Pediatric IBD
- Calprotectin Less Accurate for Isolated Ileal Crohn’s Disease
- Normative Data for Fecal Calprotectin, age 4-16 yrs
